Christina Lancioni Pediatric Research Lab
Introduction
During my childhood I witnessed the HIV-epidemic claim far too many lives across the world, as doctors and scientists raced with endless determination to learn as much as possible about this vicious virus and to develop effective treatments. As I began my journey in international health and research during medical school, I witnessed how effective and safe medications brought hope and positive change to entire communities that had been overwhelmed by HIV. I took note of how communities deeply impacted by the HIV-epidemic also struggled with other highly dangerous infectious diseases such as Tuberculosis, as well as extreme poverty and malnutrition, that conspired to worsen clinical outcomes and drive mortality. These early experiences, witnessing how science can both improve the quality of life for individuals and transform entire communities, formed the basis of my commitment to a career as a physician-scientist.
My translational immunology research program aims to delineate the underlying biologic mechanisms and consequences of immune dysregulation among highly vulnerable populations, including young infants, children living in low- and middle- income communities (LMIC), and individuals living with HIV-infection (PLWH). My lab strives to understand how age, infectious pathogens, and other environmental influences including nutrition and substance-use disorders, impact an individual’s vulnerability to severe infectious diseases, immune function, and exposure to chronic systemic inflammation. My research funding is provided by the National Institute of Health, and the Bill & Melinda Gates Foundation.
Current research program
Defining adaptive immune responses to Mtb-infection and TB disease among young children with and without HIV-exposure

Tuberculosis disease (TB), caused by Mycobacterium tuberculosis (Mtb), is a leading cause of morbidity and mortality in young children <5 yr old. The vulnerability of young children to develop TB following primary infection is not understood; this critical knowledge gap hampers efforts to develop a more effective vaccine and improved diagnostic tests for this high-risk population. Our project will comprehensively define adaptive immune responses to Mtb-exposure and TB in children < 5yr using a pediatric TB household contact study based in Kampala, Uganda, where up to 20% of children are HIV-exposed. Our approach will identify unique immunologic biosignatures driven by where the child sits on the TB disease spectrum, as well as by HIV-exposure status. Our goals are to advance pediatric global health by: 1) identification of immunologic signatures reflecting successful containment of primary Mtb infection that can serve as correlates of protective immunity for novel vaccine trials; 2) development of novel blood-based assays that discriminate between young children with TB and those whom have been exposed but successfully contained their infection. Collaborators on this NIH/NIAID funded project include: Co-PIs Dr. Catherine Stein (Case-Western Reserve University) and Dr. Ezekiel Mupere (Makerere University, Kampala, Uganda); Dr. Deborah Lewinsohn at Oregon Health & Science University; and Dr. Ryan McNamara at the Ragon Institute of MIT.
Building the evidence base for appropriate care of the sick, undernourished child in limited resource settings
Undernutrition contributes to nearly 50% of all annual deaths in children under 5 years and children living in sub-Saharan Africa and Asia continue to bear the largest burden of morbidities and mortality associated with undernutrition. Despite strong evidence of associations between undernutrition, infection and an increased risk of death among young children, the mechanisms driving this vulnerability are not understood. The Childhood Acute Illness & Nutrition Network is a group of investigators based at 9 international sites in LMIC in sub-Saharan Africa and SE Asia, who are working together to generate evidence and improved understanding of the interplay between undernutrition and acute illness during early childhood. Our over-arching goal is to identify targets for intervention that can help to avert high mortality and poor outcomes from acute illness during early childhood. Through the sponsorship of Oxford University and funding from the Bill & Melinda Gates Foundation, the CHAIN Network is sponsoring a Phase IIb clinical trial “Pancreatic Enzymes and Bile Acids: A Non-Antibiotic approach to Treat Intestinal Dysbiosis in Acutely Ill Severely Malnourished Children,” with enrollment beginning in 2021. Working with Dr. Ezekiel Mupere, a longtime collaborator, Dr. Lancioni serves as the Co-PI of the CHAIN site located in Kampala, Uganda.
The impact of substance use disorders on immune and viral dysregulation among people living with HIV (PLWH)
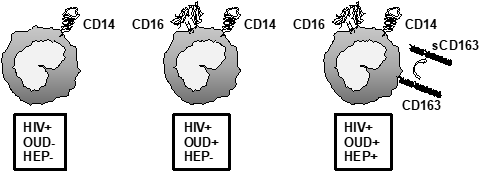
Chronic opioid use among PLWH is an on-going public health crisis. The interplay between opioids and HIV may accelerate HIV-associated immune dysregulation, chronic inflammation and coagulopathy, and predispose to AIDS and non-AIDS related mortality and morbidities. Our own published data support that opioid use is associated with advanced immune activation, alterations in monocyte phenotype, and a dysregulated cytokine responses to pathogen-associated molecular products such as lipopolysaccharide. We are now seeking to understand the mechanisms driving innate immune activation and dysregulation among PLWH who suffer from opioid-use disorder, with the goal of determining if some of the medications used to treat opioid-use disorder can reduce systemic inflammation to offer additional therapeutic benefit. Our laboratory also studies the impact of alcohol-use disorder and recreational cannabis consumption on immune function among PLWH, all in efforts to understand how to limit chronic immune activation and its negative impact on health and disease outcomes. Collaborators on this NIH/NIDA funded project include: Drs. Korthuis, Curlin, and Park at Oregon Health & Science University; and Drs. Sekaly and Pereira-Ribeiro at Emory University.
Mechanisms governing TLR-2 mediated co-stimulation of neonatal CD4+ T cells
Our research examines the ability of naïve CD4+ T cells from newborn infants to directly respond to molecules termed "Pathogen-Associated Molecular Patterns" (PAMPs) using "Toll-like Receptors)" (TLRs). Although TLRs have traditionally been described on innate antigen presenting cells, it is now recognized that T cells also use TLRs as co-stimulatory receptors. Dr. Lancioni determined that TLR-1/2 serves as a unique co-stimulatory receptor for naïve CD4+ T cells from newborn infants that allows them to become activated and differentiate into potent Th-1 effector cells productive of key pro-inflammatory cytokines, such as interferon-gamma. Currently, the laboratory is working to delineate the mechanisms governing TLR-1/2 mediated co-stimulation of naïve CD4+ T cells.