Research at Casey Eye Institute
Innovators in vision research
OHSU Casey Eye Institute has one of the top ophthalmic research programs in the country. Our scientists and clinicians have worked together to perform research studies that have revolutionized the practice of ophthalmology, and we continue to explore, innovate, and find new treatments for eye disease.
OHSU Casey Eye Institute's faculty are responsible for many of the most recent advances in ophthalmology, which have revolutionized the diagnosis and treatment of many ophthalmic conditions. Advances in optical coherence tomography, ophthalmic informatics, corneal refractive, retina and pediatric eye care have been pioneered at Casey.
Featured research programs
Patients receiving care at Casey receive the most up-to-date and innovative approaches to diagnosis and management. We are proud to be regularly ranked in the top 10 NEI-funded Eye Research Institute in the U.S. Our research faculty are making significant contributions in many areas including stem cell therapy, gene therapy, glaucoma, imaging and retinal disease.
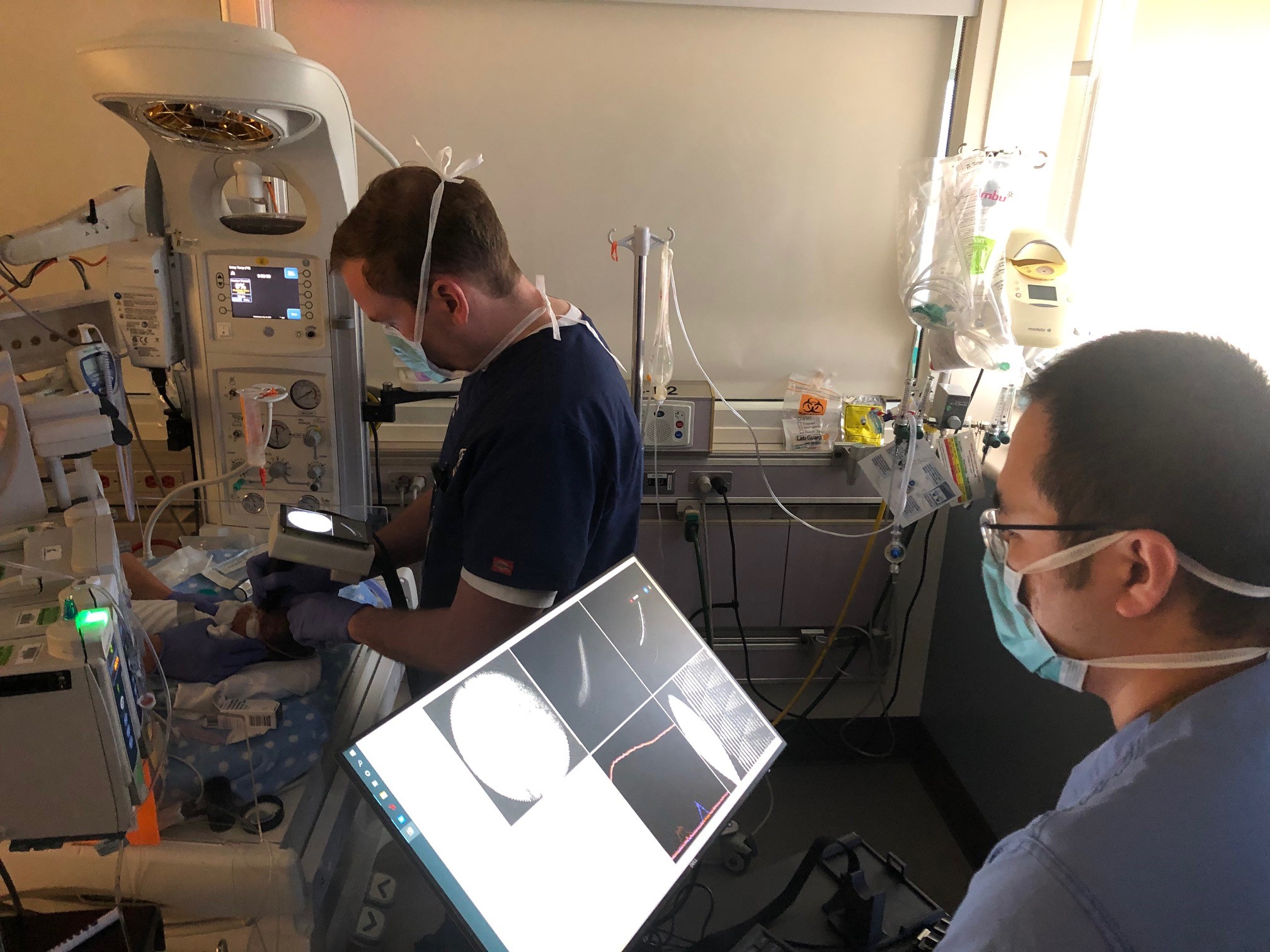
OHSU Casey Eye Institute is an international leader in applying ophthalmology informatics such as telemedicine, artificial intelligence, and big data to improve the quality and delivery of eye care. Experts are using these tools and technologies to help diagnose retinopathy of prematurity, diagnose corneal ulcers, improve clinic efficiency, and more.
Learn more
Faculty
-
- J. Peter Campbell, M.D., M.P.H.
- Edwin and Josephine Knowles Endowed Professor of Ophthalmology
-
Appointments and titles
- Professor of Ophthalmology, School of Medicine
- Edwin and Josephine Knowles Endowed Professor of Ophthalmology
-
-
Appointments and titles
- Assistant Professor of Medical Informatics and Clinical Epidemiology, School of Medicine
-
Areas of interest
- Clinic Informatics
- Data Science
- Operations Research
- Discrete Event Simulation
- Machine Learning
- Data Visualization
- Usability
-
-
- Thomas S. Hwang, M.D.
- Chief, Retina Division; Kenneth C. Swan M.D. Endowed Professor of Ophthalmology
-
Appointments and titles
- Professor of Ophthalmology, School of Medicine
- Kenneth C. Swan M.D. Endowed Professor of Ophthalmology
- Chief, Retina Division
- Vice Chair for Education
-
Areas of interest
- Graduate medical education
- Clinical informatics
- Ophthalmic imaging
-
-
Appointments and titles
- Professor of Ophthalmology, School of Medicine
- Medical Director, Casey Reading Center
-
Areas of interest
- Ocular Imaging, Image Processing, Machine Leaning, Artificial Intelligence, Glaucoma, Ophthalmology
-
This research group studies both the anterior mechanisms that affect aqueous humor outflow dynamics and the posterior mechanisms of glaucomatous optic nerve damage. The collaborative team currently holds 14 grants, representing over $24 million in total NIH funding, and seeks to use their collective research to design new and novel therapeutics for glaucoma.
Research focus areas include:
- Understanding how normal intraocular pressure regulation is lost in glaucoma and how to restore it in order to lower eye pressure.
- Understanding how normal and glaucomatous trabecular meshwork cells communicate.
- Investigating how the genes associated with hereditary glaucomas cause elevated eye pressure and developing approaches to reverse this.
- Determining how to treat the loss of intraocular pressure regulation using adult, patient-derived stem cells.
- Understanding the molecular and cellular mechanisms of glaucomatous optic nerve damage.
- Developing new therapeutic and drug delivery strategies to protect the optic nerve.
- Determining the impact of glaucoma on visual processing in retinal circuits.
- Developing new imaging technologies such as optical coherence tomography angiography to improve clinical diagnosis and monitoring of glaucoma
- Improving delivery of glaucoma care by developing remote glaucoma management systems through established community health centers.
Learn more
- Extraordinary teamwork model generates breakthroughs in glaucoma research
- Finding the other 50 percent of patients with glaucoma
- Study to investigate stem cell therapy as potential glaucoma treatment
- Investigating spectroscopic OCT to measure capillary oximetry
- Innovative imaging tools probe glaucoma’s effects
- Researchers join forces in tackling glaucoma
Faculty
-
-
Appointments and titles
- Assistant Professor of Ophthalmology, School of Medicine
-
Areas of interest
- Ocular Biomechanics
-
-
-
Appointments and titles
- Professor of Ophthalmology, School of Medicine
- Graduate Faculty, Program in Molecular and Cellular Biosciences, School of Medicine
-
-
-
Appointments and titles
- Associate Professor of Ophthalmology, School of Medicine
-
Areas of interest
- Visual Neuroscience neural circuits
- Electrophysiology
- Glaucoma
-
-
- Aiyin Chen, M.D.
- Head of the Glaucoma Division
-
Appointments and titles
- Associate Professor of Ophthalmology, School of Medicine
- Head, Glaucoma Division
- Fred P. and Joan Thompson Family Endowed Professor
-
Areas of interest
- Improving glaucoma screening using machine learning, data science, and advanced imaging technology
-
- John C. Morrison, M.D.
- Jennie P. Weeks Professor
-
Appointments and titles
- Professor of Ophthalmology, School of Medicine

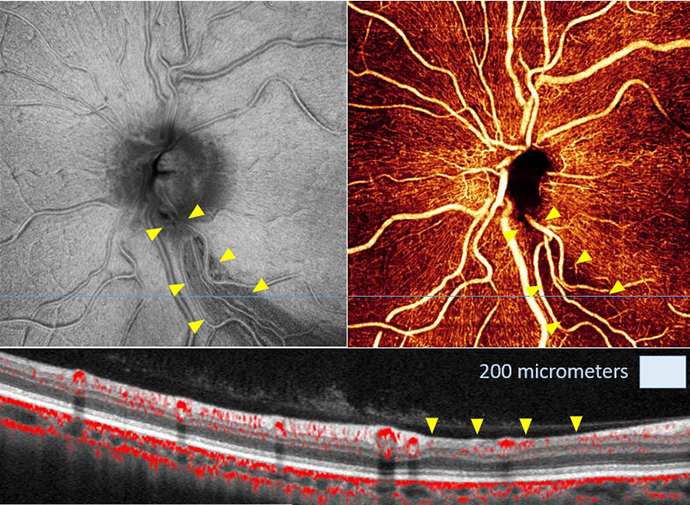
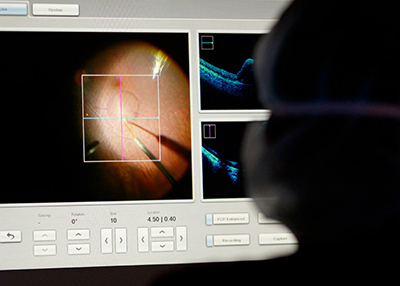
OHSU Casey Eye Institute is at the forefront of noninvasive imaging technology to detect vascular changes that may allow for treatment to prevent permanent vision loss from several eye diseases. Not only is OHSU Casey Eye Institute blazing a trail in how to use optical coherence tomography (OCT) and OCT angiography (OCT-A) clinically, but are also working to continually improve the capabilities of the technology.
The Center for Ophthalmic Optics and Lasers (COOL Lab) at Casey Eye Institute is led by David Huang, M.D., Ph.D., and is one of the leading ophthalmic imaging research groups in the world. They are dedicated to the advancement of biomedical imaging techniques in order to improve the diagnosis and treatment of eye diseases. Huang and Yali Jia, Ph.D., are now also pioneers in OCT-A, which allows doctors to map out the eye's smallest blood vessels and measure blood flow. This has potential to further transform diagnosis and treatment for common eye conditions like glaucoma, macular degeneration and diabetic eye disease.
Learn more
Faculty
-
-
Appointments and titles
- Assistant Professor of Ophthalmology, School of Medicine
- Assistant Professor of Biomedical Engineering, School of Medicine
-
-
- David Huang, M.D., Ph.D.
- Wold Family Chair in Ophthalmalogic Imaging
- Center for Ophthalmic Optics & Lasers
-
Appointments and titles
- Professor of Ophthalmology, School of Medicine
- Professor of Biomedical Engineering, School of Medicine
- Wold Family Chair in Ophthalmic Imaging
- Associate Director & Director of Research, Casey Eye Institute, School of Medicine
-
Areas of interest
- Optical coherence tomography
- Optical coherence tomographic angiography
- Biomedical optics
- Biophotonics
- Imaging
- Glaucoma
- Keratoconus
- Laser Vision Correction/LASIK/PRK
-
-
Appointments and titles
- Professor of Ophthalmology, School of Medicine
- Professor of Biomedical Engineering, School of Medicine
- Jennie P. Weeks Professor of Ophthalmology
-
Areas of interest
- Optical Coherence Tomography (OCT)
- OCT angiography
- Retinal imaging
- Artificial intellifence
-
-
-
Appointments and titles
- Associate Professor of Ophthalmology, School of Medicine
- Associate Professor, Biomedical Engineering, School of Medicine
-
Areas of interest
- optical coherence tomography; high performance computing; artificial intelligence
-
-
-
Appointments and titles
- Associate Professor of Ophthalmology, School of Medicine
-
Areas of interest
- Medical image processing
- Optical coherence tomography (OCT)
-
-
-
Appointments and titles
- Associate Professor of Ophthalmology, School of Medicine
-
Areas of interest
- Optical coherence tomography
- Medical image analysis
- Computer aided diagnosis of eye disease
-
Based on three decades of unparalleled expertise studying and caring for people with inherited eye disease, Casey Eye Institute has become a premier center for ophthalmic genetics and gene therapy research. With the establishment of the Gene Therapy Center and the Retinal Stem Cell Center, researchers at Casey Eye Institute are advancing our ability to understand and treat inherited retinal conditions faster than ever before.
Gene therapy involves doctors surgically injecting a healthy gene to replace a malfunctioning gene that causes eye disease. These remarkable treatments have successfully improved the vision of patients with legal blindness caused by inherited retinal degenerations. Because of our expertise in gene therapy, the Gene Therapy Center is one of the largest centers conducing gene therapy research, including:
- The world's first gene therapy treatment for juvenile macular degeneration.
- The world's first gene therapy treatment for Usher syndrome.
- The world's first stem cell therapy for retinitis pigmentosa.
- Being one of seven sites in the U.S. offering the first FDA-approved gene therapy for a genetic disease, Luxturna.
- The world's first first-ever CRISPR gene editing in vivo.
Learn more
- New stem cell center advances retinal disease research
- OHSU confirms first nonhuman primate model of Usher syndrome
- Nanotechnology may improve gene therapy for blindness
- A Disco in the Sky: A patient story of improved vision after gene therapy
- Gene editing clinical trial participant dreams of a future with sight
- Pioneering the first-ever CRISPR gene editing in vivo
- OHSU Casey Eye Institute grows gene and cell therapy research by performing its first stem cell therapy in the retina
- OHSU performs first-ever CRISPR gene editing within human body
- High hopes for 4-year-old’s vision after gene therapy
- Accelerating scientific research in retinal disease
Faculty
Kathleen Chirco, Ph.D.
-
-
Appointments and titles
- Assistant Professor of Ophthalmology, School of Medicine
- Member, M.D./Ph.D. Program Committee, School of Medicine
-
Areas of interest
- Inherited retinal diseases
- Retinal vascular disease
- Medical retina
- Retinal gene therapy and stem cell therapy
-
-
- Andreas K. Lauer, M.D.
- Margaret Thiele Petti and August Petti Chair of Ophthalmology
-
Appointments and titles
- Professor of Ophthalmology, School of Medicine
- Director, Casey Eye Institute, School of Medicine
- Chair, Ophthalmology, School of Medicine
- Margaret Thiele Petti and August Petti Chair of Ophthalmology, Casey Eye Institute, School of Medicine
-
-
Appointments and titles
- Professor of Ophthalmology, School of Medicine
-
Areas of interest
- Retinal Dystrophies
- Congenital/Genetic Disease
-
-
- Paul Yang, M.D., Ph.D.
- Chief, Paul H. Casey Ophthalmic Genetics Division
-
Appointments and titles
- Associate Professor of Ophthalmology, School of Medicine
- Chief, Paul H. Casey Ophthalmic Genetics Division
- Martha and Eddie Peterson Endowed Professor
-
Areas of interest
- Inherited Retinal Dystrophies, Congenital/Genetic Disease, Ocular Immunology, Autoimmune Retinopathy
-
-
Appointments and titles
- Associate Professor of Ophthalmology, School of Medicine
-
Areas of interest
- Novel therapeutics for retinal diseases
- Non-human primate models of retinal degeneration
- Gene therapy
- Cell-based therapy
-
For more than two decades, scientists at the Wold Family Macular Degeneration Center at Casey Eye Institute have contributed to key findings to improve the diagnosis, treatment and prevention of age-related macular degeneration (AMD). We continue to be a hub for leading-edge research, often working with basic scientists at Casey and other investigative groups around the world. These collaborations speed the development of new and better ways to manage AMD.
Faculty
-
-
Appointments and titles
- Professor of Ophthalmology, School of Medicine
-
Specialty
- Ophthalmology
- Macular Degeneration
- Retina and Vitreous Disease
- Retina Surgery
-
-
- Christina J. Flaxel, M.D., FARVO
- Bula Buck Arveson and Charles C. Arveson Professor of Macular Degeneration Research
-
Appointments and titles
- Professor of Ophthalmology, School of Medicine
- Bula Buck Arveson and Charles C. Arveson Professor of Macular Degeneration Research
- Director of the Wold Family Macular Degeneration Center
-
Specialty
- Ophthalmology
- Macular Degeneration
- Retina and Vitreous Disease
- Retina Surgery
-
- Thomas S. Hwang, M.D.
- Chief, Retina Division; Kenneth C. Swan M.D. Endowed Professor of Ophthalmology
-
Appointments and titles
- Professor of Ophthalmology, School of Medicine
- Kenneth C. Swan M.D. Endowed Professor of Ophthalmology
- Chief, Retina Division
- Vice Chair for Education
-
Specialty
- Ophthalmology
- Macular Degeneration
- Retina and Vitreous Disease
- Retina Surgery
-
-
Appointments and titles
- Professor of Ophthalmology, School of Medicine
-
Specialty
- Ophthalmology
- Macular Degeneration
- Retina and Vitreous Disease
-
-
-
Appointments and titles
- Associate Professor of Ophthalmology, School of Medicine
-
Specialty
- Ophthalmology
- Comprehensive Ophthalmology
-
Clinical trials
Clinical trials are research studies involving volunteer participants in which scientists learn whether a new medication or treatment is safe and effective in people. Trials are conducted in phases, often over a number of years.
Patients who participate in clinical trials have access to the newest medications that are being studied for potential approval. We are grateful for patients who participate in clinical trials for contributing to the evaluation of new treatments that may benefit future generations.
Casey conducts clinical trials focusing on several areas, including age-related macular degeneration, glaucoma, neuro-ophthalmology and gene therapy treatments for inherited eye conditions.
Casey Reading Center
The Casey Reading Center is made up of a team of talented physicians, scientists, and staff who work with clinical trial sponsors to ensure that trials are of the highest quality and allow for the best possible scientific insights into the results.
Since we started operating in 2009, we have developed operating procedures for clinical trials that comply with both domestic and international regulatory requirements and are considered "best in class" by clinical trial experts.
Drug registry
Casey helps to support and coordinate the National Registry of Drug-Induced Ocular Side Effects, with support from the American Academy of Ophthalmology.
The registry, founded in 1976, is an international clearinghouse of information on how drugs, chemicals, herbs and herbal supplements can cause adverse events in eye conditions or vision.
The registry's goal is to offer eye professionals information on early signals of an adverse eye reaction.
Research training programs
OHSU Casey Eye Institute offers several training programs for students and clinicians who are interested in pursuing vision research opportunities, including two prestigious vision research training programs funded by the National Institutes of Health.