Ryals Lab

Lab introduction
The Ryals lab develops and evaluates novel therapeutics for inherited retinal degenerations in small animal models. We maintain a colony of many different mouse and rat models of inherited retinal degeneration. Working with these animals, we employ gene-based, cell-based and neuroprotective therapies to slow or mitigate disease progression.
Renee Ryals, Ph.D., was born and raised in Daytona Beach, Florida. She received her BS in Chemistry and her PhD in Molecular Cell Biology and Clinical and Translational Science at the University of Florida. Her dissertation focused on expanding the utility of AAV gene therapy for inherited retinal dystrophies by generating dual AAV vectors for the delivery of large transgenes. After graduation, Renee moved to Portland, Oregon for her post-doctoral training with Dr. Mark Pennesi where she evaluated neuroprotective agents and their ability to slow retinal degeneration in a light-induced retinopathy model. Currently, Renee is an Associate Professor in the Department of Ophthalmology working alongside both Dr. Pennesi and Dr. Sahay in a combined effort to develop lipid nanoparticle gene therapies for retinal degeneration. In her free time, Dr. Ryals enjoys community and personal development. She enjoys finding opportunities to utilize her process-oriented facilitation and conflict resolution skills.
Dr. Ryals is also affiliated with the Molecular & Medical Genetics department at OHSU. She has collaborations with Dr. Hiroyuki Nakai, Dr. Melanie Gillingham and Dr. Susan Hayflick. Dr. Ryals is also affiliated with the Department of Neuroscience, Oregon National Primate Research Center. She is collaborating with Dr. Martha Neuringer and Dr. Trevor McGill and leading studies that evaluate lipid nanoparticles in the nonhuman primate retina.
Selected projects
Gene therapy
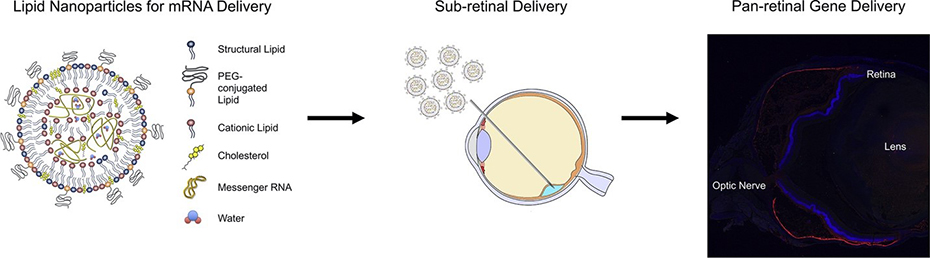
We are developing lipid nanoparticle (LNP) delivery systems that efficiently transfect retinal cells, especially photoreceptors and RPE. Our goal is to develop LNP-mediated gene editing platforms for inherited retinal degenerations.
Cell therapy
We are working with multiple companies to evaluate the efficacy of transplanted cells (RPE, photoreceptors, neuroprogenitors) to slow degeneration in the Royal College of Surgeons rats and mouse models of inherited retinal degenerations. These studies aid in the IND application process and bring novel therapeutics one step closer to the clinic.
Laboratory members
Allison Curtis – Allie is a Senior Research Assistant in the Ryals laboratory. She graduated from Pacific Lutheran University in Tacoma, WA with a B.S. in Biology. In the Ryals lab, she coordinates lipid nanoparticle mediated gene editing experiments. She assists with: animal colony maintenance & surgery, live animal imaging, tissue processing, microscopy, and in vitro work. When not in the lab, Allie spends her time reading, attempting to keep her many houseplants alive, and trying out different kinds of art.
Sebastian Arrizabalaga - Sebastian is a Senior Research Assistant in the Ryals laboratory. He graduated from Reed College in Portland, OR, with a B.A. in Chemistry. His expertise includes tissue processing, immunohistochemistry, confocal microscopy, and animal colony maintenance. Sebastian’s hobbies include tabletop gaming, 3d printing, and cooking.
Grace Su - Grace Li-Na Su (they/them) is a PhD student in the Ryals laboratory. They graduated from Doane University in Crete, NE in 2021 with a B.S. in Biomedical Engineering. Their expertise include rodent visual assessments, tissue processing, immunohistochemistry, and confocal microscopy. Grace's hobbies include dying their hair fun colors, tabletop and video games, and walking their cat at the farmers market.
Nash Redmayne, MS - Nash is a Senior Research Associate and lab manager for the Ryals laboratory. He earned his B.S. in Biology and Chemistry at University of Washington and his MS in Biochemistry and Molecular Biology at OHSU. Prior to joining Casey Eye Institute, Nash spent 10 years investigating the impact of epigenetic dysregulation on multiple disease states in human and non-human primate models. His expertise includes next-generation sequencing, single-cell whole genome amplification, RNA fluorescence in-situ hybridization, and laser microscopy. When he’s not in the lab, Nash can be spotted in his native PNW searching for undiscovered waterfalls or tending his backyard forest garden.
Selected publications
Preformed Vesicle Approach to LNP Manufacturing Enhances Retinal mRNA Delivery
May 13, 2024
Yulia Eygeris, Michael I. Henderson, Allison G. Curtis, Antony Jozić, Jonathan Stoddard, Rene Reynaga, Kathleen R. Chirco, Grace Li-Na Su, Martha Neuringer, Andreas K. Lauer, Renee C. Ryals, Gaurav Sahay
Thiophene-based lipids for mRNA delivery to pulmonary and retinal tissues
Mar 12, 2024
Yulia Eygeris, Mohit Gupta, Jeonghwan Kim, Antony Jozic, Milan Gautam, Jonas Renner, Dylan Nelson, Elissa Bloom, Adam Tuttle, Jonathan Stoddard, Rene Reynaga, Martha Neuringer, Andreas K. Lauer, Renee C. Ryals, Gaurav Sahay
A G1528C Hadha knock-in mouse model recapitulates aspects of human clinical phenotypes for long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency
Dec 2023
Garen Gaston, Shannon Babcock, Renee Ryals, Gabriela Elizondo, Tiffany DeVine, Dahlia Wafai, William Packwood, Sarah Holden, Jacob Raber, Jonathan R. Lindner, Mark E. Pennesi, Cary O. Harding, Melanie B. Gillingham
Glial, Neuronal, Vascular, Retinal Pigment Epithelium, and Inflammatory Cell Damage in a New Western Diet–Induced Primate Model of Diabetic Retinopathy
2023
Tailoi Chan-Ling, Ping Hu, Sergio Li Calzi, Jeff Warner, Nasir Uddin, Mariana DuPont, Martha Neuringer, Paul Kievit, Lauren Renner, Jonathan Stoddard, Renee Ryals, Michael E. Boulton, Trevor McGill, Maria B. Grant
Lipid nanoparticles with PEG-variant surface modifications mediate genome editing in the mouse retina
Dec 2023
Milan Gautam, Antony Jozic, Grace Li Na Su, Marco Herrera-Barrera, Allison Curtis, Sebastian Arrizabalaga, Wayne Tschetter, Renee C. Ryals, Gaurav Sahay
Peptide-guided lipid nanoparticles deliver mRNA to the neural retina of rodents and nonhuman primates
Jan 2023
Marco Herrera-Barrera, Renee C. Ryals, Milan Gautam, Antony Jozic, Madeleine Landry, Tetiana Korzun, Mohit Gupta, Chris Acosta, Jonathan Stoddard, Rene Reynaga, Wayne Tschetter, Nick Jacomino, Oleh Taratula, Conroy Sun, Andreas K. Lauer, Martha Neuringer, Gaurav Sahay
Spontaneous allelic variant in deafness–blindness gene Ush1g resulting in an expanded phenotype
Aug 2023
Vladimir Vartanian, Jocelyn F. Krey, Paroma Chatterjee, Allison Curtis, Makayla Six, Sean P.M. Rice, Sherri M. Jones, Harini Sampath, Charles N. Allen, Renee C. Ryals, R. Stephen Lloyd, Peter G. Barr-Gillespie
An improved protocol for generation and characterization of human-induced pluripotent stem cell-derived retinal pigment epithelium cells
Dec 16, 2022
Harshini Surendran, Lalitha Soundararajan, Vijay Bhaskar Reddy K, Janavi Subramani, Jonathan Stoddard, Rene Reynaga, Wayne Tschetter, Renee C. Ryals, Rajarshi Pal
Increasing the Efficacy of Gold Nanorod Uptake in Stem Cell-Derived Therapeutic Cells
2022
Grant W. Marquart, Jonathan Stoddard, Karen Kinnison, Felicia Zhou, Richard Hugo, Renee Ryals, Scott Shubert, Trevor J. McGill, Marilyn R. Mackiewicz
A ketogenic & low-protein diet slows retinal degeneration in rd10 mice
2020
Renee C. Ryals, Samuel J. Huang, Dahlia Wafai, Claire Bernert, William Steele, Makayla Six, Shasank Bonthala, Hope Titus, Paul Yang, Melanie Gillingham, Mark E. Pennesi