The new center explores exciting possibilities for treating inherited retinal diseases that lead to blindness
Casey Eye Institute has a long history of advances in gene and cell-based therapies. The founder of its ophthalmic genetics division, Richard Weleber, M.D., laid the foundation decades ago for a leading center for gene replacement, gene augmentation and stem cell therapy trials, including several first-in-human trials.
From genetic testing to stem cells
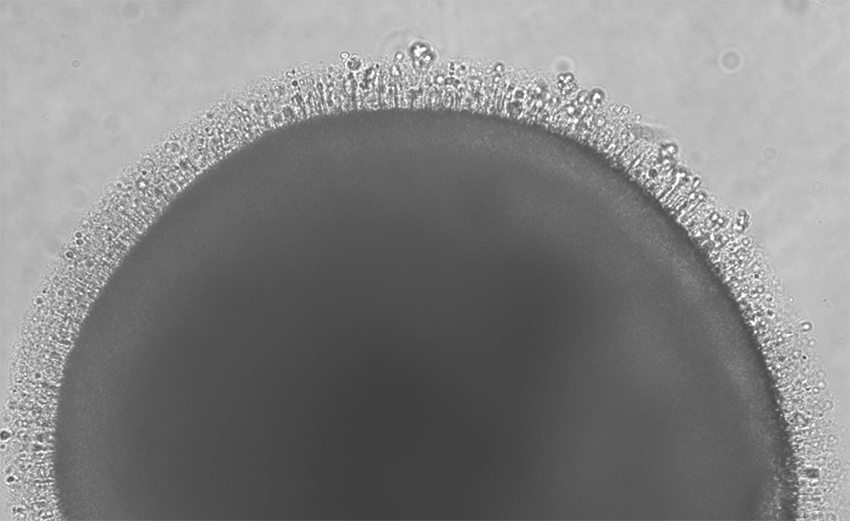
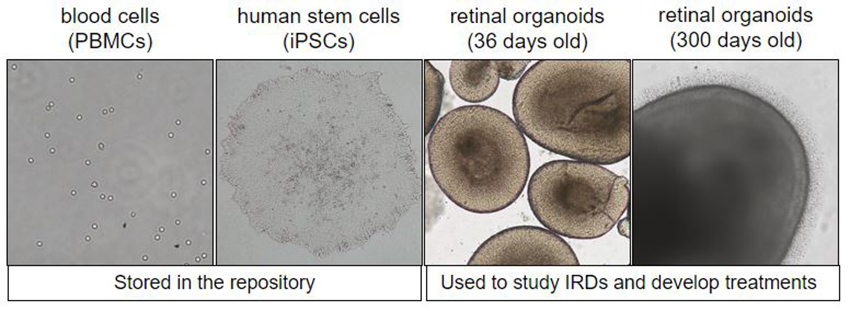
The new Retinal Stem Cell Center is building on this foundation. The center is a repository for stem cells grown from patients with inherited retinal diseases, or IRDs. The cells are induced pluripotent stem cells, or IPSCs, derived from donated blood samples. They are used to grow retinal organoids in vitro – essentially, miniature retinas in Petri dishes.
Casey Eye Institute is well positioned to collect samples of a wide range of IRDs. Mark Pennesi, M.D., Ph.D., is now head of the Paul H. Casey Ophthalmic Genetics Division. “We have a large patient population, and we see people from infancy up to 100 years of age,” he says. “Because genetic diseases are lifelong, we also follow patients for many years.”
Years of research and support pay off for patients
Pennesi and others at Casey Eye Institute were instrumental in obtaining FDA approval for voretigene neparvovec-ryzl (Luxturna; Spark Therapeutics, Philadelphia, PA) and treating some of the first patients. The therapy helps restore vision in patients with mutations in both alleles of RPE65 who have enough healthy retinal cells.
The development of Luxturna has given hope to people with a wide range of retinal diseases. “It took genetic specialists 10 to 15 years to develop this treatment,” says Casey ophthalmic geneticist Paul Yang, M.D., Ph.D. “Today, we are doing the basic research that will lead to future therapeutics.”
Casey researchers are creating the repository as a global resource. “Our hope is to create a library available to researchers worldwide,” Yang says. “If a colleague is interested in a particular mutation, we can send them the cells to be grown and studied, leveraging the resources of Casey Eye Institute.”
Progress against inherited retinal disease owes much to private philanthropy in creating this type of infrastructure, Pennesi says. “The Casey family endowed the Paul H. Casey Genetics Division, $50 million was raised to build the new Elks Children’s Eye Clinic that our division calls home, and other donors are now generously supporting the Retinal Stem Cell Center.”
The benefits of “mini-retinas”
It takes about 100 days to create a single retinal organoid, as Casey Eye Institute scientist Kathleen Chirco, Ph.D., carefully nurtures the cells with the help of a technician. However, hundreds of these mini-organs can be developed at once. Pennesi explains the value of this approach.
“Treating patients in clinical trials is very expensive,” he says. “We can only treat a small number of patients, using therapies that are a sure bet, because we don’t want to do any harm. But with hundreds of retinal organoids, we can potentially try hundreds of different drugs. It extends our ability to try new things with no risk to patients.”
“Another exciting feature of the Retinal Stem Cell program is that it is scalable,” Chirco says. “Patients have benefited from what was already developed at Casey, and now we are building the next generation of therapies.”
The Retinal Stem Cell Center also reduces the need for animal research. “Traditionally, efficacy is demonstrated in animal models as we take an investigational product from pre-clinical research to clinical trials,” says Casey ophthalmic geneticist Lesley Everett, M.D., Ph.D. “Eventually, we need to demonstrate safety in non-human primates.”
However, this type of research is extremely expensive. Using retinal organoids allows researchers to demonstrate that a treatment could work in human eyes. Pennesi says, “Stem cell trials may not allow you to completely eliminate animal research, but it can replace much of it while providing more reliable results because the stem cells are human.”
New paths to treating all stages of retinal disease
The number of patients in need of treatment for vision loss due to IRDs helped drive Casey’s ophthalmic genetics team to establish the stem cell center.
“Gene therapy and gene editing treatments [such as with Luxturna] are most valuable for people who are losing their vision now,” says Pennesi. “Unfortunately, they don’t help those who have lost it already. We need additional options in the regenerative therapy space, and this is where stem cells come in. The center gives us a new infrastructure so we can develop treatment options for retinal disease at every stage.”
Stem cells are also the best way to study certain diseases. “There are some [diseases] for which there are no other good models,” says Yang. “For example, a common mutated gene in retinitis pigmentosa is called EYS. Unfortunately, mice do not have that gene, so we cannot study it in mice. We can study some retinal diseases in dogs, but we are dependent on mutations that naturally occur in certain dog breeds.”
The ultimate personalized medicine
Actually replacing diseased retinal cells could be possible one day. “There are certainly challenges to overcome, but in theory, we could correct a mutation, grow healthy retinal cells from the patient’s own cells, and then reintroduce them,” Pennesi says. This would reduce the chances for rejection inherent in using cells from a universal stem cell line.
The Retinal Stem Cell Center offers Casey’s ophthalmic geneticists new ways to study inherited retinal diseases and potential treatments. By studying IRDs at the organoid level, they hope one day to be able to offer sight-saving treatments to patients who have few options today.