Casey Reading Center
Providing comprehensive, high quality and insightful image reading services
The Casey Reading Center (CRC) is a comprehensive ophthalmic reading center providing custom support to clinical sites with data acquisition, endpoint development, custom analysis for Sponsors, and insightful image reading services. It offers a secured image transmission platform and sophisticated functional and imaging analysis to ensure the highest quality and insight from clinical trials.
The CRC has extensive experience providing detailed guidance to clinical sites, certifying study technicians, and acquiring trial files/ images with appropriate quality standards based on the study's requirements to collect insightful information and ensure data integrity.
Contact us
The Casey Reading Center looks forward to working with sponsors from the inception of a clinical trial through its conclusion to provide the best experience possible in obtaining verified data. Thank you for your interest in the Casey Reading Center.
Please send new inquiries to:
Shobana Aravind, Ph.D.,
Associate Director of the Casey Reading Center
Email: aravind@ohsu.edu
General support: readingcenter@ohsu.edu
Phone: 503 418-2189
Quick Links
- Custom clinical support and guidance
- Custom analysis
- Services available
- Optical coherence tomography angiography
- Visual Field: 3D modelling and analysis
- Structure-function overlay and analysis
- Custom OCT biomarker quantification
- Key personnel
- Contact us
What we do
Expertise
Custom clinical support and guidance
- Clinical site manual and guidance for image/file acquisition.
- Custom grid development and installation for perimetry file acquisition.
- Site technician's software training.
- Site certification for staff and equipment.
Custom analysis
- Custom endpoints development and analysis.
- Manual of procedures development based on custom endpoints.
- AI assisted quality image and file acquisition.
- AI and automated reading services.
Services available
Reading Center services offered using OCTA
Retinal fluid quantification
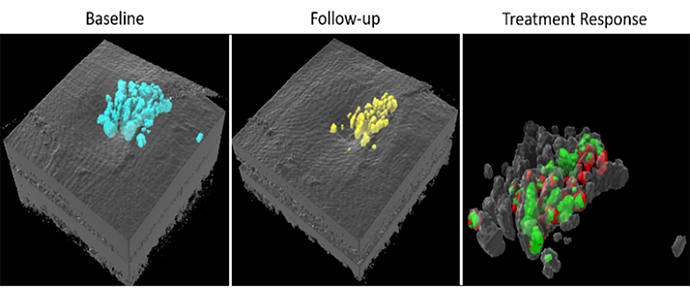
Our deep learning based fluid segmentation algorithm uses OCTA information together with structural OCT data to obtain accurate fluid volume measurements in 3D at high resolution (blue), and can be used to monitor treatment response (yellow: fluid imaged during follow up; red: retained fluid, green: successfully treated fluid).1
1 Guo Y, et al. Transl Vis Sci Technol. 2020;9(2)
Plexus-specific non-perfusion area quantification
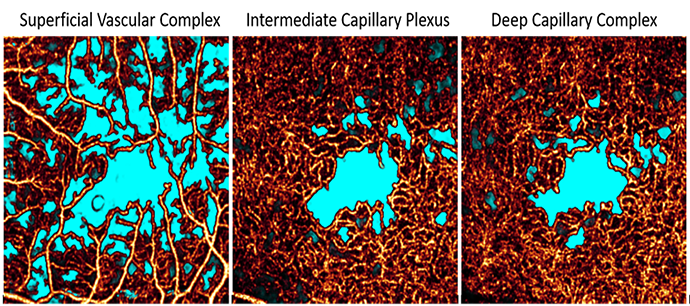
Our novel deep learning based non-perfusion area detection algorithm is resilient to shadow artifacts and can segment this pathology in any plexus and in wide-field images. This approach has been used by the diabetic retinopathy clinical research network (DRCR.Net)3.
3 Guo Y, et al. Ophthalmol Sci. 2021; 1(2)
Choroidal neovascularization vessel area
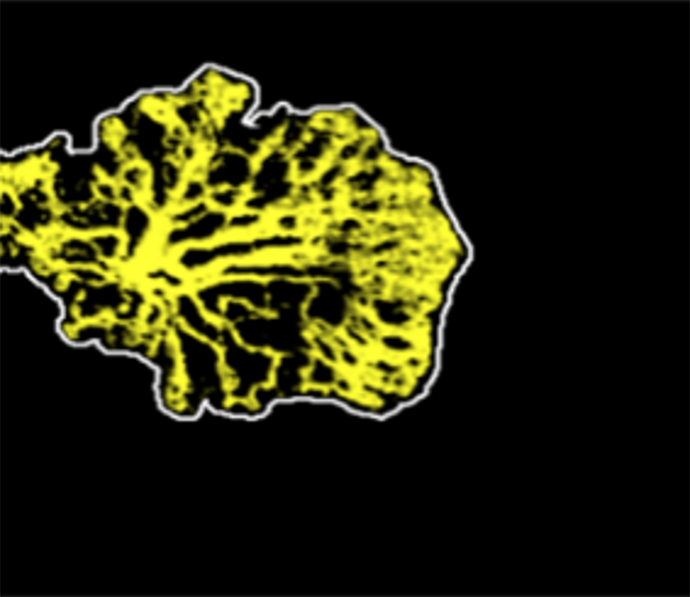
Choroidal neovascularization can be imaged even before exudation occurs in age-related macular degeneration (AMD). Such lesions can be automatically segmented using deep learning.4
4 Wang J, et al. Biomed Opt Express. 2020; 11(2)
Retinal artery and vein differentiation and assessment
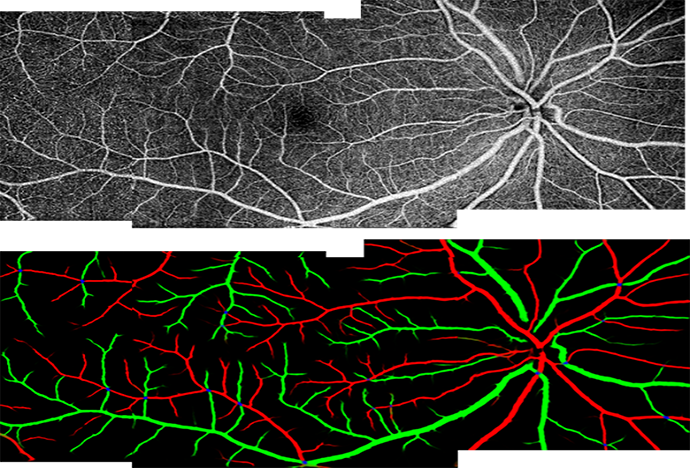
Our cutting-edge approach based on deep learning can distinguish arteries (red) from veins (green) in both macular, optic disc, and wide-field images. With this capability, common OCTA metrics such as vessel density and tortuosity can be measured separately for arteries and veins, illuminating disease pathophysiology.5
5 Gao M, et al. Ophthalmol Science. 2022
Artificial intelligence disease grading and activation map
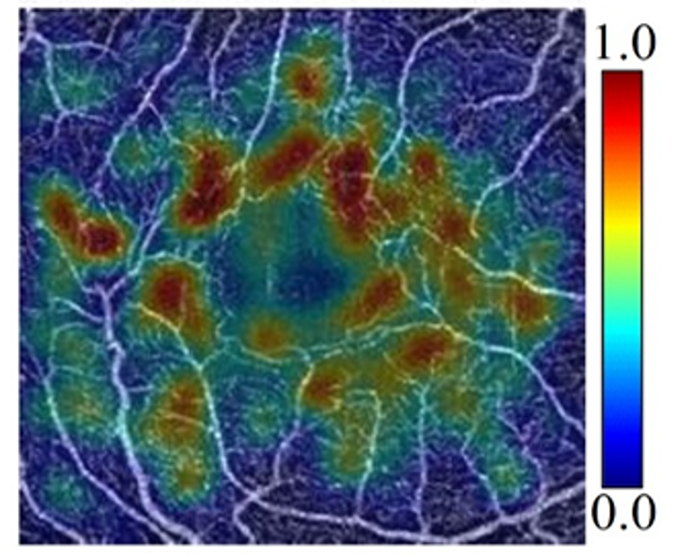
Diabetic retinopathy severity is determined by noting the presence and the extent of several pathologic features. But with deep learning methods diabetic retinopathy can also be staged directly from OCTA data volumes without appealing to specific biomarkers. Our innovative model is not only highly accurate, but also produces novel biomarker activation maps that highlight pathologic features and can help clinicians verify algorithm output.6
6 Zang P, et al. IEEE Trans Biomed Eng. 2021;68(6)
Projection-resolved OCTA algorithm
Projection-resolved OCTA algorithm removes projection artifacts volumetrically providing clean images of the deepest capillaries in the retina.2
2 Wang J, et al. Biomed Opt Express. 2017; 8(3)
Key personnel for OCTA services
Yali Jia, Ph.D. – Technical Director
Li Qin Gao, M.D. – Chief Grader
Jie Wang, Ph.D. – Principal Engineer
Denzil Romfh, O.D. – OCTA Coordinator Lead
Providing visual field modeling and analysis
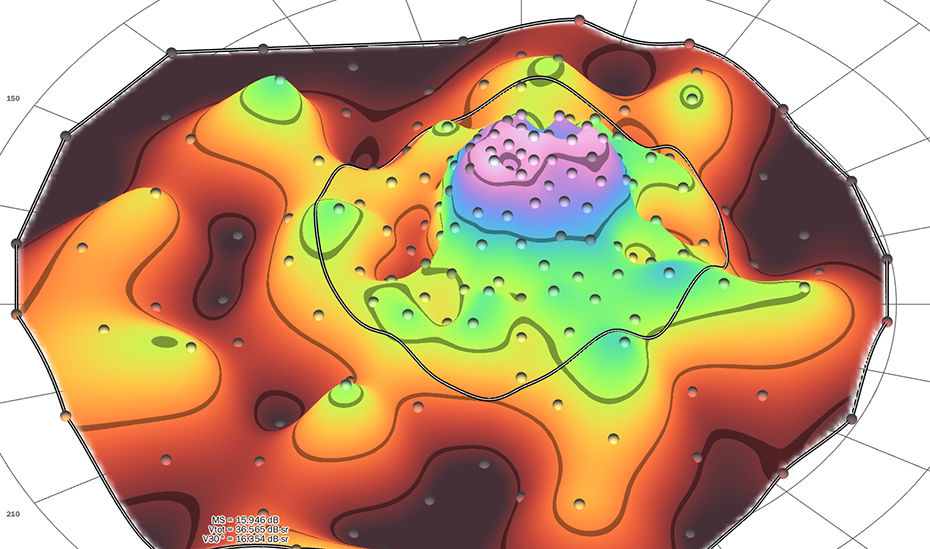
The Casey Reading Center (CRC) draws on more than 30 years of Casey Eye Institute's experience in perimetry, provides OHSU proprietary Visual Field Modeling and Analysis (VFMA),1 the most advanced topographic analysis of the visual field.
VFMA renders 3D surface models of the hill of vision (HOV) and its defects, providing quantitative functional measures.
CRC has provided VFMA data for many clinical trials targeting various inherited retinal diseases.2 We also provide custom visual field testing grid patterns based on the need of the trial.
—
1. Weleber RG et al. Transl Vis Sci Technol. 2015 Apr 28; 4(2):14.
2. Smith TB, et al. PLoS One. 2016 Apr 13;11(4).
Structure-function overlay and analysis using VFMA Overlay Tool
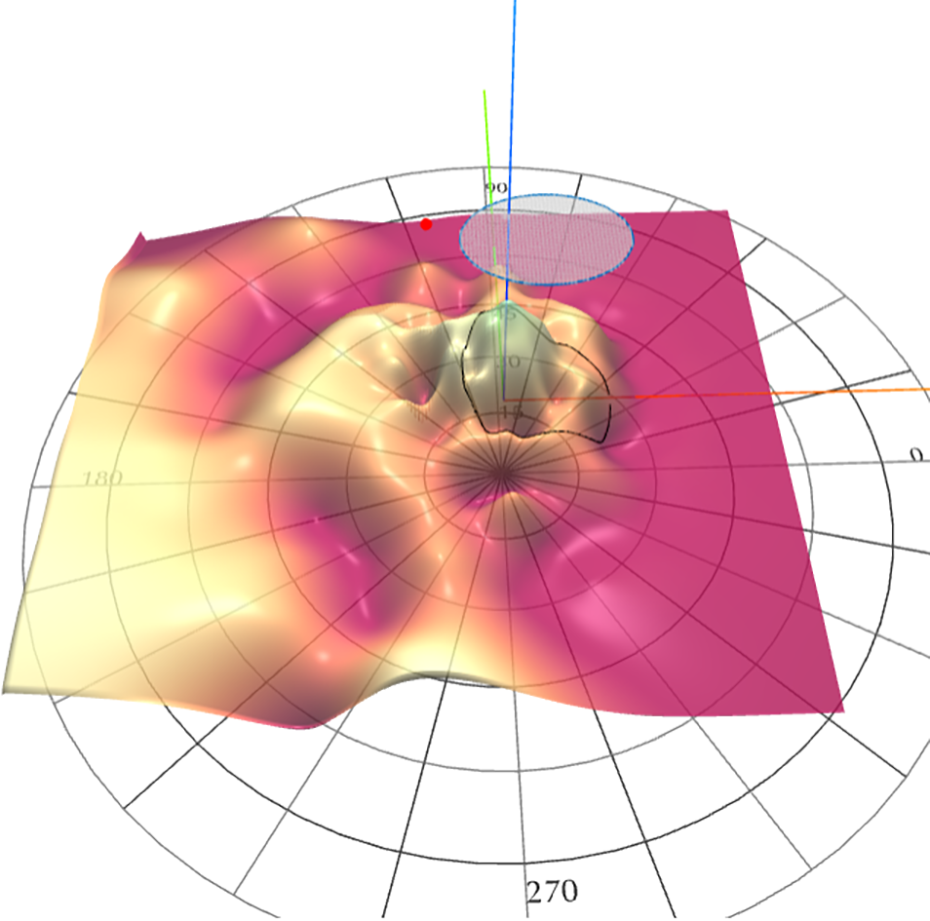
The CRC has developed VFMA Overlay Tool, a proprietary novel software for Structure-Functions Assessment for clinical trials.
3D topographic surface analysis of HOV superimposed over corresponding OCT en face image allows the CRC to visualize precisely registered spatial relationship between structure and function as well as assess the longitudinal changes in function at a region of interest in the retina.
One of the useful applications of the Overlay software is to assess retinitis pigmentosa cases after gene therapy using viral vectors. HOV overlay allows measuring the functional changes within and/or outside the ‘bleb’ (vector injection site).
Custom OCT biomarker quantification includes:
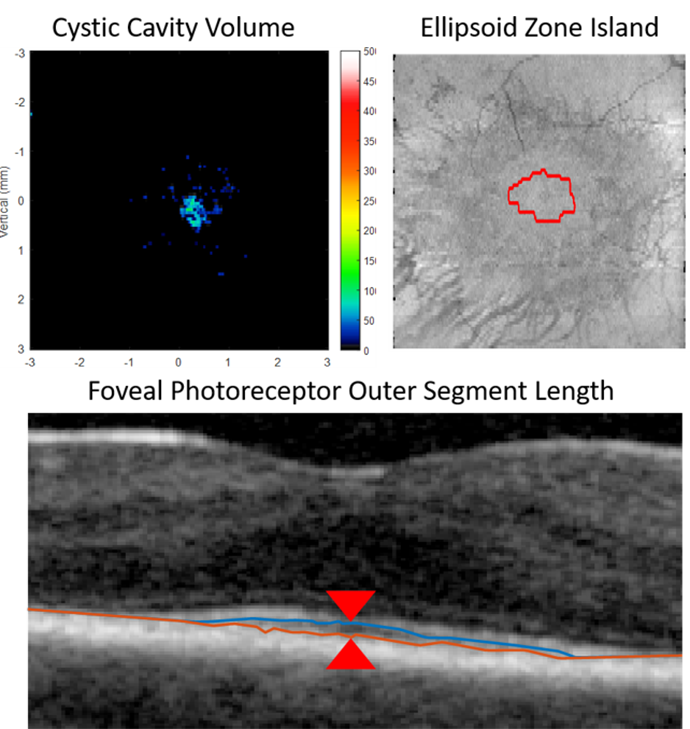
- Retinal fluid quantification
- Ellipsoidal zone
- Outer segment length
Calculating cystic cavity volume, ellipsoid zone boundary detection, and quantification of foveal photoreceptor outer segment length.
Key Personnel
Founders

David J. Wilson, M.D.
Founder
Dr. Wilson is Professor Emeritus of Ophthalmology at OHSU and former director of the Casey Eye Institute. Dr. Wilson’s research involves study of the pathophysiology of eye disease. He has a particular interest in oncology, inherited retinal disease and gene- and cell-based therapies.

Richard G. Weleber, M.D.
Founder
Dr. Weleber was a Professor of Ophthalmology at OHSU Casey Eye Institute and chief of the ophthalmic genetics division.
Key Personnel

Hiroshi Ishikawa, M.D.
Medical Director
Dr. Ishikawa is a Professor of Ophthalmology at OHSU Casey Eye Institute. He has over 25 years of research experience in image processing for ocular imaging devices (i.e. optical coherence tomography (OCT), ultrasound biomicroscopy (UBM)) and machine learning applications in clinical ophthalmology.

Shobana Aravind, Ph.D., BS Pharm, CCRP
Director of Operations
Dr. Aravind has over 15 years of experience in clinical research administration, conduct of clinical trials and pharmaceutical product development. Data quality and data management have been her particular areas of expertise.

Thomas Hwang, M.D.
Investigator
Dr. Hwang specializes in retinal diseases and vitreoretinal surgery. His research areas of interest include clinical informatics, and ophthalmic imaging.

Yali Jia, Ph.D.
Technical Director, OCT Angiography Services
Yali Jia, PhD is an Associate Professor of Ophthalmology and Biomedical Engineering, Jennie P. Weeks Professor of Ophthalmology and associate director of the Center for Ophthalmic Optics and Lasers at OHSU.
Her research interests include optical coherence tomography (OCT), OCT angiography, and retinal imaging.

Paul Yang, M.D., Ph.D.
Investigator
Dr. Paul Yang is chief of the Paul H. Casey Ophthalmic Genetics Division and Martha and Eddie Peterson Endowed Professor at OHSU Casey Eye Institute. He also has special interest in gene therapy associated uveitis (GTU), autoimmune retinopathy, and cataract surgery in patients with inherited retinal degenerations. The focus of his research is to better understand the mechanisms of immune-mediated damage in inherited retinal degenerations and autoimmune retinopathy.
Data quality
E-systems and quality
The CRC offers interactive and secure web-based software for the management of clinical trials. It is remotely accessible, allowing easy upload of data and image fi les from clinical sites while allowing real time access for the CRC, sponsors and clinical research organizations. Due to its accessibility, we have taken great pains to ensure the data housed within our database is electronically secured. The reading center software, database and server are FDA compliant for electronic records, according to 21 CFRP art 11, and we follow the FDA guidance put forth on General Principles of Software Validation; Final Guidance for Industry and FDA Staff. The data center and servers are maintained by a specialized team of information technology professionals. Data is backed-up daily and stored off-site. Servers are protected by SSL encryption, ongoing virus and malware surveillance, as well as scanning at the time of upload.
Publications
Publications from the Casey Reading Center
The sample list of publications below were supported by the Casey Reading Center.
Assessing the impact of image quality on deep learning classification of infectious keratitis
Adam Marcus Hanif; Venkatesh Prajna; Prajna Lalitha; Erin NaPier; Maria Parker; Peter Steinkamp; Jeremy Keenan; J. Peter Campbell; Xubo Song; Travis Redd
Investigative Ophthalmology & Visual Science June 2023, Vol.64, 1078.
Three-year safety results of SAR422459 (EIAV-ABCA4) gene therapy in patients with ABCA4-associated Stargardt disease: an open-label dose-escalation phase I/IIa clin.
Maria A Parker, Laura R Erker, Isabelle Audo, Dongseok Choi, Saddek Mohand-Said, Kastytis Sestakauskas, Patrick Benoit, Terence Appelqvist, Melissa Krahmer, Caroline Ségaut-Prévost, Brandon J. Lujan, Ambar Faridi, Elvira N. Chegarnov, Peter N Steinkamp, Cristy Ku, Mariana Matioli da Palma, Pierre-Olivier Barale, Sarah Ayelo-Scheer, Andreas Lauer, Tim Stout, David J Wilson, Richard G Weleber, Mark E. Pennesi, José Alain Sahel, Paul Yang,*
Am J Ophthalmol. 2022 August ; 240: 285–301.
The natural history of choroideremia; progressive loss of visual function and retinal structure.
David G Birch, Paul S Bernstein, Ian M MacDonald, Timothy Stout, David S Liao, Kirsten G Locke, Yi Zhai, Audra K Miller, Jenny Holt, Adia Jackson Leung, Somayeh Honarmand, Paul Jordan, Ulrich F O Luhmann, David H Kirn, Peter J Francis; Invest.
Ophthalmol. Vis. Sci. 2020;61(7):1908.
Reliability of spectral-domain OCT ellipsoid zone area and shape measurements in retinitis pigmentosa.
Smith TB, Parker MA, Steinkamp PN, Romo A, Erker LR, Lujan BJ, Smith N.
Trans Vis Sci Tech. 2019;8(3):37,
Prospective Evaluation of Patients With X-Linked Retinoschisis During 18 Months.
Pennesi ME, Birch DG, Jayasundera KT, Parker M, Tan O, Gurses-Ozden R, et al.
IOVS. 2018;59(15).
Importance of Focus in OCT Angiography.
Tomlinson A, Hasan B, Lujan BJ.
Ophthalmol Retina. 2018 Jul;2(7):748-749. doi: 10.1016/j.oret.2018.01.012. Epub 2018 Feb 22.
Repeatability and Longitudinal Assessment of Foveal Cone Structure in CNGB3-associated Achromatopsia.
Christopher S. Langlo, Laura Erker, Maria Parker, Emily J. Patterson, Brian Higgins, Phyllis Summerfelt, Moataz Razeen, Frederick T. Collison, Gerald A. Fishman, Christine N. Kay, Jing Zhang, Richard G. Weleber, Paul Yang, David Wilson, Mark E. Pennesi, Byron L. Lam, John Chiang, Jeffrey D. Chulay, Alfredo Dubra, William W. Hauswirth, Joseph Carroll, and the ACHM-001 study group.
Retina 2017 Oct;37(10):1956-1966.
Residual Foveal Cone Structure in CNGB3-associated Achromatopsia.
Christopher S. Langlo, Emily J. Patterson, Brian Higgins, Phyllis Summerfelt, Moataz Razeen, Laura Erker, Maria Parker, Frederick T. Collison, Gerald A. Fishman, Christine N. Kay, Jing Zhang, Richard G. Weleber, Paul Yang, David Wilson, Mark E. Pennesi, Byron L. Lam, John Chiang, Jeffrey D. Chulay, Alfredo Dubra, William W. Hauswirth, Joseph Carroll, and the ACHM-001 study group.
IOVS. Aug 2016.
Test-Retest Variability of Functional and Structural Parameters in Patients with Stargardt Disease Participating in the SAR422459 Gene Therapy Trial.
Parker MA, Choi D, Erker LR, Pennesi ME, Yang P, Chegarnov EN, et al.
Transl Vis Sci Technol [Internet]. 2016;5(5):10.
Structure-Function Modeling of Optical Coherence Tomography and Standard Automated Perimetry in the Retina of Patients with Autosomal Dominant Retinitis Pigmentosa.
Smith TB, Parker MA, Steinkamp PN, Weleber RG, Smith N, Wilson DJ.
Lewin AS, editor. PLoS One [Internet]. 2016 Feb 4;11(2):e0148022
VFMA: Topographic analysis of sensitivity data from fullfield static perimetry.
Weleber RG, Smith TB, Peters, D, et al.
Trans Vis Sci Tech. 2015;4(2):14
Results at 2 years after gene therapy for RPE65- deficient leber congenital amaurosis and severe early-childhood-onset retinal dystrophy.
Weleber RG, Pennesi ME, Wilson DJ, Kaushal S, Erker LR, Jensen L, et al.
Ophthalmology. 2016;123:1606–20.
News
News and links
News and links
Learn more about the work being done by the Casey Reading Center:
- Video: Innovative Technology Series – Casey Reading Center
- Winners of 2022 Biomedical Innovation Program awards address significant clinical problems, featuring Yali Jia, Ph.D.
- Dr. Wilson awarded Preuss Distinguished Alumni Award from SoM
- Yali Jia, Ph.D., elected National Academy of Inventors Senior Member
- OHSU experts tapped for national Artificial Intelligence initiative