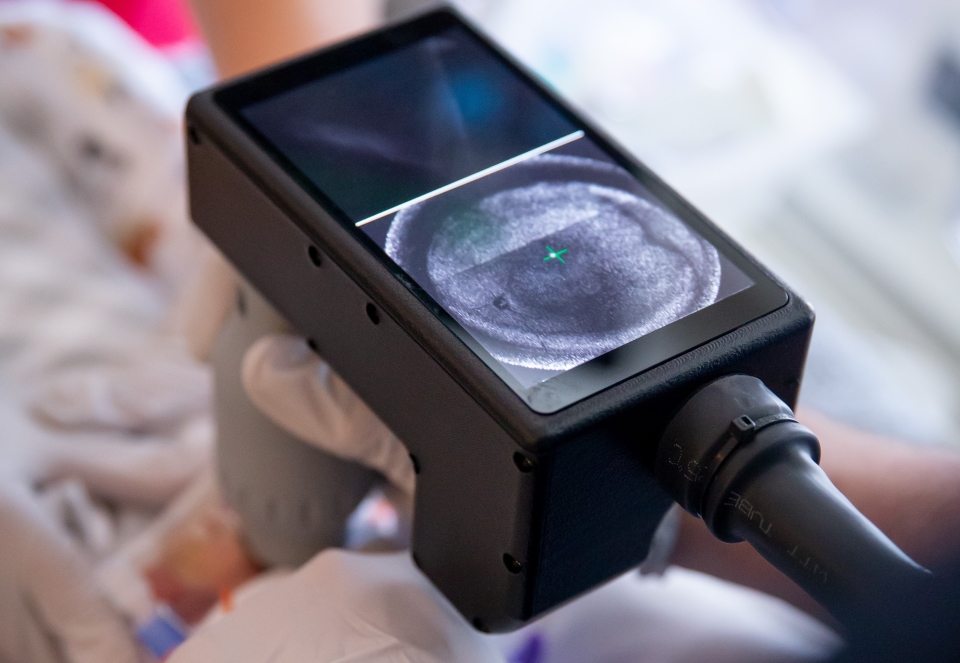
New system improves retinopathy of prematurity diagnoses
OHSU Casey Eye Institute has developed the world's first handheld ultra-widefield OCT system for diagnosing retinopathy of prematurity in premature infants. Following decades of leadership in ROP research and artificial intelligence applications, the institute is addressing a critical gap in pediatric retinal care.
J. Peter Campbell, M.D., M.P.H., Edwin and Josephine Knowles Endowed Professor of Ophthalmology, and Yifan Jian, Ph.D., associate professor of ophthalmology and biomedical engineering at Oregon Health & Science University, have conducted more than 3,000 eye exams using this investigational device, the only system of its kind in the world.
Closing the pediatric imaging gap
Optical coherence tomography has revolutionized adult retinal care, enabling clinicians to visualize cellular-level pathology across nearly every retinal disease. Due to technological limitations, pediatric patients have not benefited equally.
"When we see kids, the standard of care is still drawing a picture with pencil and paper," said Campbell. "We're essentially two decades behind."
ROP is the leading cause of childhood blindness worldwide. In the United States, approximately 500 babies go blind from ROP annually—blindness that is largely preventable. Globally, the number reaches 50,000 children per year.
Traditional ROP examination presents substantial challenges. The procedure requires physically manipulating the infant's eye with a scleral depressor, a process that can cause bradycardia and desaturation in vulnerable premature infants. Diagnosis relies on subjective assessment, leading to significant variation in treatment decisions.
"The eye exam in babies is really hard," Campbell explained. "Your field of view is small, so you spend several minutes creating a mental mosaic. With the new OCT system, we see everything in one second."
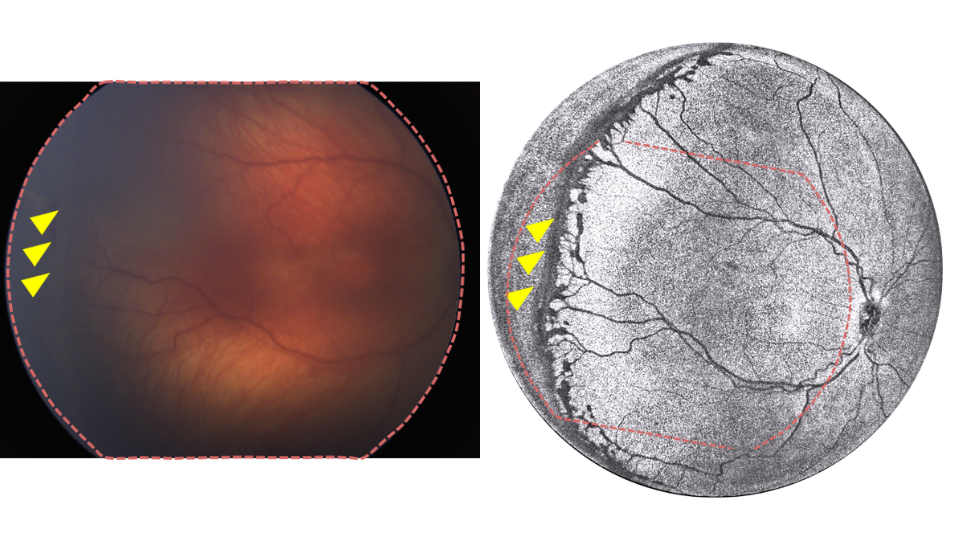
Technical innovation through team science
The Casey Eye Institute system represents a comprehensive engineering solution built from the ground up. Operating at speeds of 400 kHz to 800 kHz, the device captures images of awake, non-sedated infants. The field of view extends to 140 degrees, enabling visualization to the ora serrata without a scleral depressor.
The team has designed custom optical components, including specialized lenses and fibers to minimize light loss, and developed computational systems using rapid graphics processing units for real-time image processing. Campbell notes the result is "better than any adult OCT on the market in the U.S. right now in terms of the field of view. There's actually nothing available like it, either for adults or kids."
Jian attends clinical rounds in the NICU, observing real-world challenges and evolving the hardware design based on clinical feedback. This bench-to-bedside approach reflects a model of team science established at OHSU Casey Eye Institute by Michael F. Chiang, M.D., now director of the National Eye Institute.
"Being able to work with ophthalmologists and test what we've developed in real life, and to see the impact immediately—that's what drives our research," said Jian.
From subjective to quantitative diagnosis
The ultra-widefield OCT system enables a fundamental shift from subjective staging to objective quantification. Rather than relying on hand-drawn sketches, the system provides quantifiable measurements of ridge thickness, volume, and anatomical detail, as well as with permanent documentation.
Integrated AI algorithms process and segment images in real time. The system can be used to detect disease autonomously for telemedicine screening. Severity scoring can be standardized, reducing diagnostic variation.
"Our long-term goal is to integrate the AI into the OCT, so that you get a great picture and a severity score that makes us all speak the same language," Campbell said.
The AI component is built on OHSU Casey Eye Institute's previous work. In 2020, the i-ROP DL (deep learning) system received FDA Breakthrough Device designation for diagnosing clinically significant ROP.
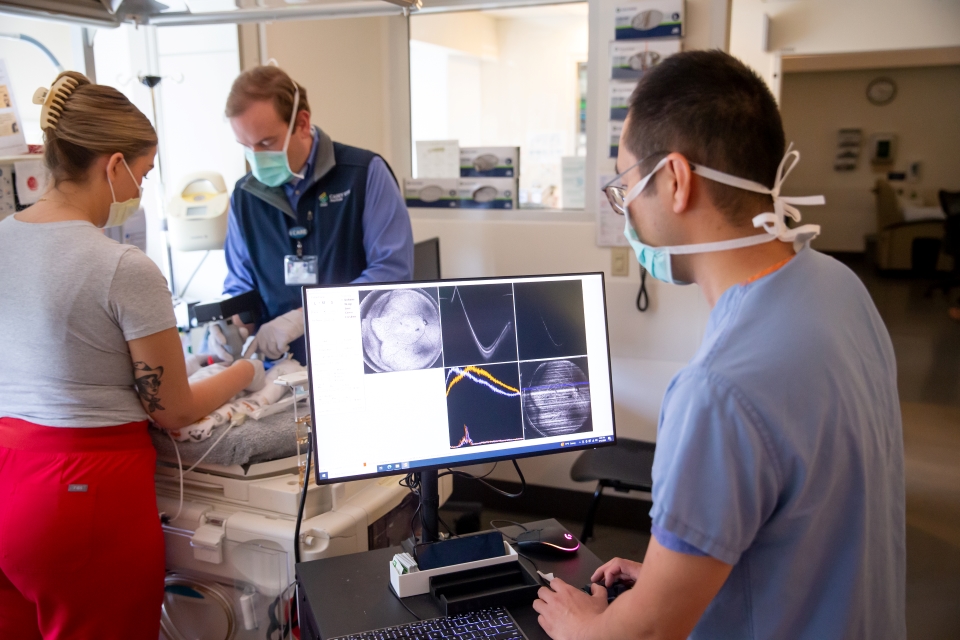
The path to clinical implementation
Both the imaging system and integrated AI technology are progressing through FDA approval, with commercial availability anticipated in the next year or so through an OHSU startup company.
"A few years ago, I switched my focus from writing papers to solving the most interesting problem: moving technology out of the lab and having it used clinically," Campbell said.
The team is developing a second-generation camera system that maintains the wide field of view and speed while reducing the cost for global applications. Orbis International, the world's largest ophthalmic NGO, has invested in the company through venture philanthropy, recognizing that addressing global ROP blindness requires a telemedicine solution.
"Our goal is to get a camera into every NICU in the world over the next five to 10 years," Campbell said.
Expanding applications
Through collaboration with colleagues at Casey Eye Institute, the ultra-widefield OCT system is being adapted for retinoblastoma screening, uveal melanoma monitoring, and intraoperative surgical guidance. Campbell received an innovation award at a Cleveland Clinic neonatology conference in 2024.
Continuing the legacy of ROP leadership
OHSU Casey Eye Institute has maintained a leadership position in ROP research for more than four decades. Earl Palmer, M.D., conducted the first clinical trial in ROP at the institution. Michael Chiang redefined the international classification of ROP approximately five years ago.
"Oregon has punched above its weight in terms of ROP care for forty years," Campbell said. "If you look at top 10 institutions for ROP impact, we are certainly in the top three or four."
The Campbell-Jian collaboration is continuing this legacy, addressing the longstanding clinical challenges of ROP and blazing a path towards giving about 50,000 children each year the gift of sight.