Center advances research into rare diseases
Growing new organs in the laboratory is a longtime goal of medical research. The option of replacing diseased or damaged organs with healthy human tissue could change countless lives. Supported by philanthropy, Casey Eye Institute’s Retinal Stem Center is a few steps closer to making that dream a reality for people with inherited retinal diseases (IRDs). IRDs are rare, no treatments are available to date, and these diseases eventually lead to vision loss.
Creating “mini-retinas”
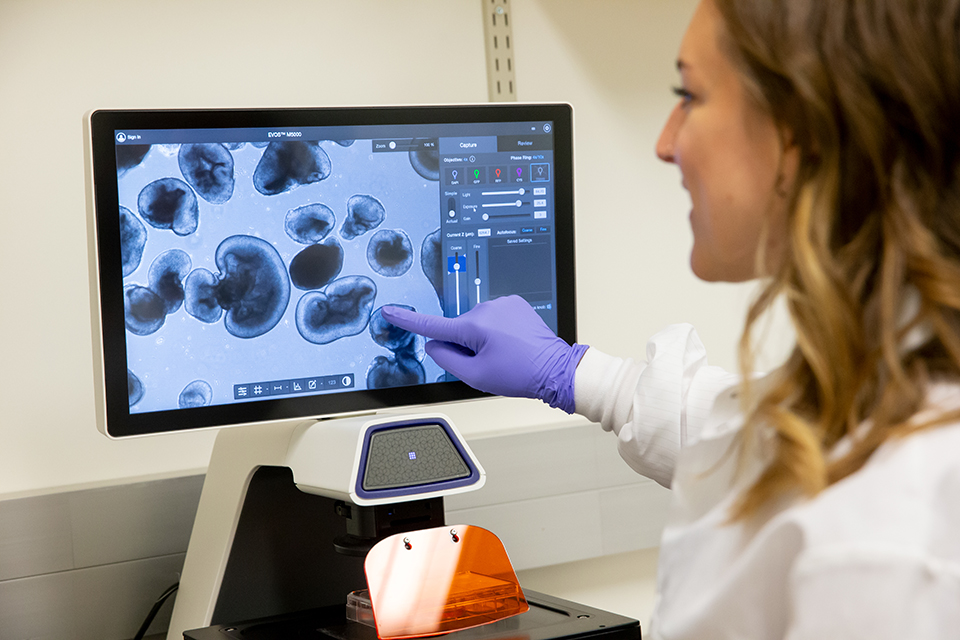
Starting with blood samples donated by Casey patients living with inherited retinal diseases (IRDs), the Retinal Stem Cell Center’s scientists are creating stem cells that grow into tiny retinas in about 240 days. Called retinal organoids, they are being used to study IRDs and develop new treatments.
“It’s incredible. We give them these cues, and the cells do everything themselves. They assemble themselves into three-dimensional spheres and then keep growing and maturing,” says Katie Chirco, Ph.D., assistant professor of ophthalmology at Casey Eye Institute.
Donor investment is essential to this project, both because IRDs are rare and because Casey scientists are using such revolutionary techniques. Stem cell research is less likely than some other projects to receive funding from major granting agencies such as the National Institutes of Health. Up to now, this has meant little hope for people with IRDs. However, Casey researchers have never hesitated to take on big tasks if it means saving sight.
A solid investment
Casey Eye Institute has the facilities, staff and technology needed to develop treatments for complex conditions. It also has a long history of research into genetic and rare eye diseases. Years ago, the Center for Ophthalmic Genetics was developed into a leading center for gene replacement, stem cell therapy trials and other innovations, including several clinical trials that were the first ever done in humans.
Today, Casey researchers conduct more gene therapy clinical trials than almost any location worldwide. Mark Pennesi, M.D., Ph.D., head of the Paul H. Casey Ophthalmic Genetics Division, and his colleagues helped gain approval from the US Food and Drug Administration for a drug called voretigene neparvovec-rzyl (Luxturna®), which can restore sight in people with a specific genetic eye disease. The Retinal Stem Cell Center’s work with organoids could lead to many more sight-saving treatments.
“The thing that amazes me is the ingenuity that these researchers demonstrate, and their dedication to coming up with new and better ways to help humanity,” says Andreas K. Lauer, M.D., director of the OHSU Casey Eye Institute, chair and professor for the Department of Ophthalmology.
A scaled-up approach to research
In the Retinal Stem Cell Center, hundreds of mini-retinas can be developed at once. Working on a larger scale increases researchers’ efficiency. It is also safer and less costly than traditional research methods.
“Treating patients in clinical trials is very expensive,” says Paul Yang, M.D., Ph.D., an ophthalmic geneticist at Casey Eye Institute. “We can only treat a small number of patients, using therapies that are a sure bet, because we don’t want to do any harm. But with hundreds of retinal organoids, we can potentially try hundreds of different drugs. The organoids extend our ability to try new things with no risk to patients.”
They also reduce the need for animal research. The effectiveness of new eye treatments is often demonstrated in non-human primates with normal retinas, but using retinal organoids can replace much of this testing. In addition, the mini-retinas are created using human cells from patients with IRDs, so the results of research are more reliable and potentially better at predicting how well treatments will work in clinical trials.
In fact, only human cells will do for studying some diseases, including IRDs. “A common mutated gene in retinitis pigmentosa is called EYS,” says Lesley Everett, M.D., Ph.D., a Casey ophthalmic geneticist. “Unfortunately, mice do not have that gene, so we cannot study it in mice. We can study some retinal diseases in dogs, but we are dependent on mutations that naturally occur in certain dog breeds.” The Retinal Stem Cell Center’s work allows this type of study in human tissue.
Phase One: A dream becomes reality
The researchers’ determination and the generosity of Casey’s donors has made Phase One of the Retinal Stem Cell Center’s work a reality. A research team is in place and successfully growing 3D retinas for study. Ten stem cell lines are now available for basic research.
With additional funding from the National Eye Institute and the Foundation Fighting Blindness, the Casey team is studying Usher syndrome type 1B and Bardet-Biedl syndrome, two inherited diseases that cause vision loss.
Phase Two: Expanding to do more
Now, Phase Two of Retinal Stem Cell Center development is ready to launch. When it is fully funded, the center will be able to expand by:
- Developing retinal organoids for even more inherited diseases
- Studying dozens of diseases a year, instead of two or three
- Testing multiple types of treatments
- Creating a collection of tissues with specific genetic mutations that can be shared with other labs around the world for study and research collaboration.
To do this much, the Center will need to add scientists, technicians, and dedicated equipment – from incubators and sterile work stations for stem cells and organoids to microscopes and freezers. A sequencing machine can speed the process of developing new treatments, and robotic assistants can help with repetitive tasks to free human technicians for other work.
The future of IRD treatment
Will the “mini-retinas” developed at Casey’s Retinal Stem Cell Center ever be used to replace a diseased retina entirely? Theoretically, this is possible, Pennesi says. Using a patient’s own cells to create the new retina would reduce the chance of rejection for the transplanted tissue.
For now, this remains a goal, not a reality. But researchers at the Retinal Stem Cell Center will continue to innovate with the goal of changing as many lives as possible through sight-saving treatments. Their hard work, and the compassion of those who support it, are creating new possibilities for people with inherited retinal disease.