Han Lab
Welcome to the Han Laboratory located at the Oregon National Primate Research Center at Oregon Health & Science University. Our team focuses on contraception and fertility, with an emphasis on nonhormonal contraception development, and improving the side-effect profile of existing contraceptives.
About us
Our team’s research vision focuses on understanding fertility regulation in the lower reproductive tract. We have clinical, translational and basic science projects focused on developing and testing new contraceptive drugs, particularly those that work through preventing sperm ascension in the reproductive tract. Our lab uses the nonhuman primate (NHP) as a model for evaluating nonhormonal contraceptives. We are also interested in endometrial changes in response hormonal and nonhormonal drugs as well as systemic disease.
Lab Highlights
- Development of conditionally reprogrammed endocervical cell cultures
- In vitro profiling of endocervical cell mucus production
- Detailed studies of CFTR and other epithelial ion channels in the endocervix
- Novel clinical trials to evaluate cervical mucus changes
- Single-cell profiling of endometrial changes in response to progestin-only contraception
In addition to lab work, our team partners with the women’s health research unit at OHSU where we conduct translational clinical trials in contraception and gynecology
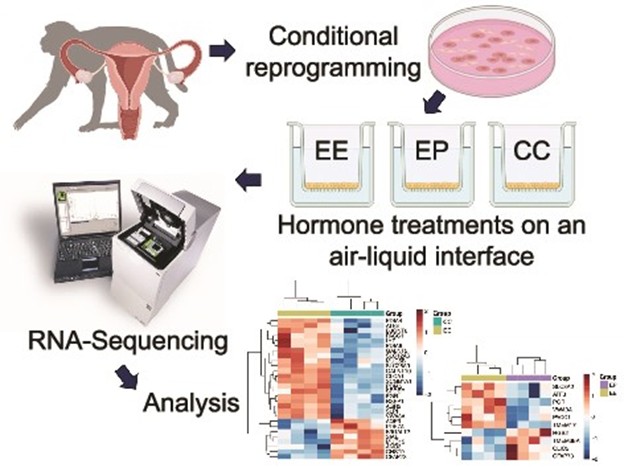
Rapp K, Wei S, Roberts M, Yao S, Fei SS, Gao L, Ray K, Wang, A, Godiah R, Han L. Transcriptional profiling of mucus production in rhesus macaque endocervical cells under hormonal regulation. Biology of Reproduction 2024;111:1045–55.
Endocervical mucus production is a key regulator of fertility throughout the menstrual cycle. With cycle-dependent variability in mucus quality and quantity, cervical mucus can either facilitate or block sperm ascension into the upper female reproductive tract. This study seeks to identify genes involved in the hormonal regulation of mucus production, modification, and regulation through profiling the transcriptome of endocervical cells from the non-human primate, the rhesus macaque (Macaca mulatta). We treated differentiated primary endocervical cultures with estradiol (E2) and progesterone (P4) to mimic peri-ovulatory and luteal-phase hormonal changes. Using RNA-sequencing, we identified differential expression of gene pathways and mucus-producing and mucus-modifying genes in cells treated with E2 compared to hormone-free conditions and E2 compared to E2-primed cells treated with P4. We pursued differential gene expression analysis on RNA-sequenced cells. Sequence validation was done using quantitative PCR (qPCR). Our study identified 158 genes that show significant differential expression in E2-only conditions compared to hormone-free control and 250 genes that show significant differential expression in P4-treated conditions compared to E2-only conditions. From this list, we found hormone-induced changes in transcriptional profiles for genes across several classes of mucus production, including ion channels and enzymes involved in post-translational mucin modification that have not previously been described as hormonally regulated. Our study is the first to use an in vitro culture system to create an epithelial cell–specific transcriptome of the endocervix. As a result, our study identifies new genes and pathways altered by sex steroids in cervical mucus production. In vitro hormonal regulation of mucus production, modification, and secretion was profiled using primary epithelial endocervical cells.
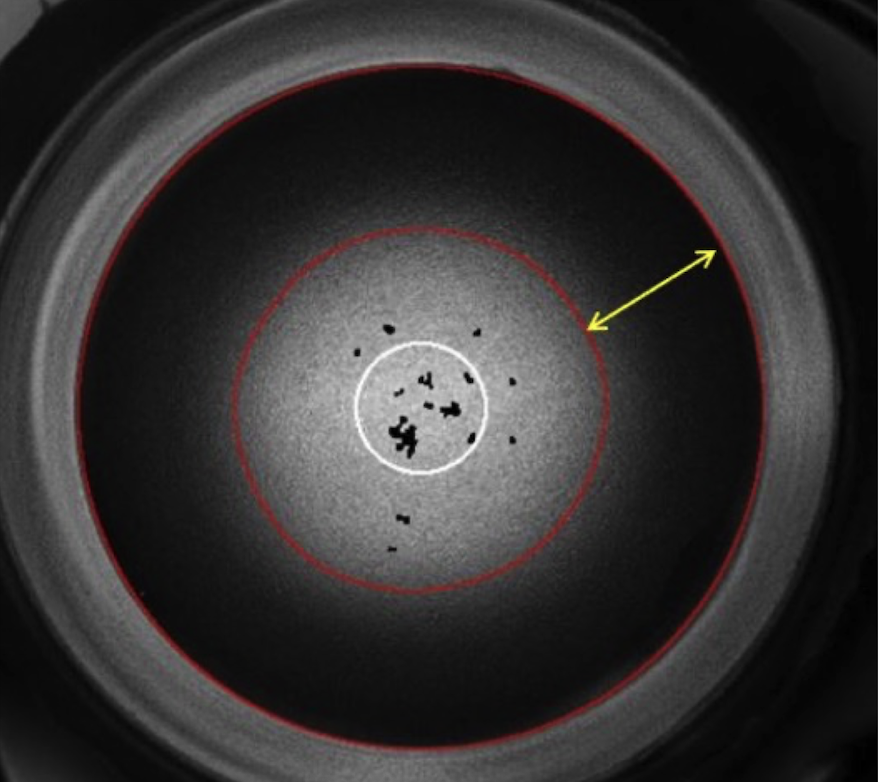
Looney RJ, Roberts M, Markovetz M, Godiah R, Yao S, Golgotiu K, Wei S, Celluci C, Han L. In vitro inhibition of the CFTR ion channel in the Macaca mulatta cervix thickens cervical mucus. Biology of Reproduction 2025:ioaf103
Cervical mucus changes throughout the menstrual cycle in response to hormonal fluctuations, regulating access of sperm and pathogens to the reproductive tract. Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) is an anion channel that plays a critical role in mediating epithelial mucus secretions. Primary endocervical cells obtained from rhesus macaques Macaca mulatta were cultured using conditional reprogramming and treated with vehicle controls or CFTR inhibitors. In order to measure changes in hydration and viscosity of secreted mucus, we adapted two airway mucus assays, airway surface liquid and particle-tracking microrheology, for our endocervical culture system. Endocervical cells treated with CFTR inhibitors demonstrated dehydrated, thicker mucus secretions compared to controls in both assay outputs. Our studies suggest that CFTR may be an important mediator of fertility changes and provide experimental evidence for the infertility phenotype seen in women with cystic fibrosis. Additionally, assays developed in these studies provide new endpoints for assessing cervical mucus changes in vitro.
Han L, Roberts M, Luo A, Wei S, Slayden OD, Macdonald KD. Functional Evaluation of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) in the Endocervix. Biology of Reproduction 2022:ioac090. PMCID: PMC9476216
The cystic fibrosis transmembrane conductance regulator (CFTR) is an apical membrane chloride/bicarbonate ion channel in epithelial cells. Mutations in CFTR cause cystic fibrosis, a disease characterized by thickened mucus secretions and is associated with subfertility and infertility. CFTR function has been well characterized in vitro and in vivo in airway and other epithelia studies. However, little is known about CFTR function in the cervix in health and its contribution to cyclic regulation of fertility from endocervical mucus changes. Contributing to this research gap is the lack of information on the effect of sex steroid hormones on CFTR expression in cervical epithelial cells across the menstrual cycle. Herein, we demonstrate the hormonal regulation of CFTR expression in endocervical cells both in vitro and in vivo, and that conditionally reprogrammed endocervical epithelial cells can be used to interrogate CFTR ion channel function. CFTR activity was demonstrated in vitro using electrophysiological methods and functionally inhibited by the CFTR-specific inhibitors inh-172 and GlyH-101. We also report that CFTR expression is increased by estradiol in the macaque cervix both in vitro and in vivo in Rhesus macaques treated with artificial menstrual cycles. Estrogen upregulation of CFTR is blocked in vivo by cotreatment with progesterone. Our findings provide the most comprehensive evidence to date that steroid hormones drive changes in CFTR expression. These data are integral to understanding the role of CFTR as a fertility regulator in the endocervix.
- Han L, Andrews W, Wong K, Jensen JT. Conditionally Reprogrammed Macaque Endocervical Cells Retain Steroid Receptor Expression and Produce Mucus. Biol of Reproduction 2020 May 26;102(6):1191-1202. PMID: 32232331; PMCID: PMC7253786.
- Park D, Reddy AP, Wilmarth PA, Jensen JT, Han L. Mucus secretions from a conditionally reprogrammed primary endocervical cell culture. F&S Science 2022;3:159–65. PMCID: PMC9947459
- Han L, Park D, Reddy A, Wilmarth P, Jensen JT. Comparing Endocervical Mucus Proteome of Humans and Rhesus Macaques. Proteomics Clinical Applications Proteomics Clin Appl. 2021 Jul; 15(4): e2100023 PMCID: PMC8653767
- Han L, Roberts M, Luo A, Wei S, Slayden OD, Macdonald KD. Functional Evaluation of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) in the Endocervix. Biology of Reproduction 2022:ioac090. PMCID: PMC9476216
- Looney RJ, Roberts M, Markovetz M, Godiah R, Yao S, Golgotiu K, Wei S, Celluci C, Han L. In vitro inhibition of the CFTR ion channel in the Macaca mulatta cervix thickens cervical mucus. Biology of Reproduction 2025:ioaf103.
- Rapp K, Wei S, Roberts M, Yao S, Fei SS, Gao L, Ray K, Wang, A, Godiah R, Han L. Transcriptional profiling of mucus production in rhesus macaque endocervical cells under hormonal regulation. Biology of Reproduction 2024;111:1045–55.
- Han L, Padua E, Hart, K, Edelman A, Jensen JT. Comparing cervical mucus changes in response to an oral progestin or estrogen withdrawal in ovarian suppressed women: a clinical pilot The European Journal of Contraception & Reproductive Health Care. 2019 Jun;24(3):209-215. PMID: 31066303; PMCID: PMC6638556.
- Han L, Padua E, Edelman A, Jensen JT. Appraising cervical mucus: a new approach to evaluating contraceptives. The European Journal of Contraception & Reproductive Health Care. 2018;23:78–83. PMID: 29457758.
- Han L, Creinin MD, Hemon A, Glasier A, Chen MJ, Edelman A. Mechanism of action of a 0.075 mg norgestrel progestogen-only pill 2. Effect on cervical mucus and theoretical risk of conception. Contraception 2022;112:43–7. PMID: 35367204
- Han L, Taub R, Jensen JT. Cervical mucus and contraception: what we know and what we don’t. Contraception 2017;96:310–21. PMID: 28801053