Two Researchers, Two Approaches: AI-Powered Tools Transform Disease Management
At Casey Eye Institute, two scientists are harnessing artificial intelligence to revolutionize glaucoma care. Ou "Tomy" Tan, Ph.D., and Hiroshi Ishikawa, Ph.D., are developing distinct AI-driven approaches that promise to transform how clinicians diagnose and monitor this sight-threatening disease.
Dr. Tan: Personalizing glaucoma diagnosis through OCT
Dr. Tan, associate professor of ophthalmology at Oregon Health & Science University, is working to enable earlier diagnosis of glaucoma before significant damage occurs. Glaucoma is often called the “silent thief of sight” because vision loss can begin long before patients notice symptoms.
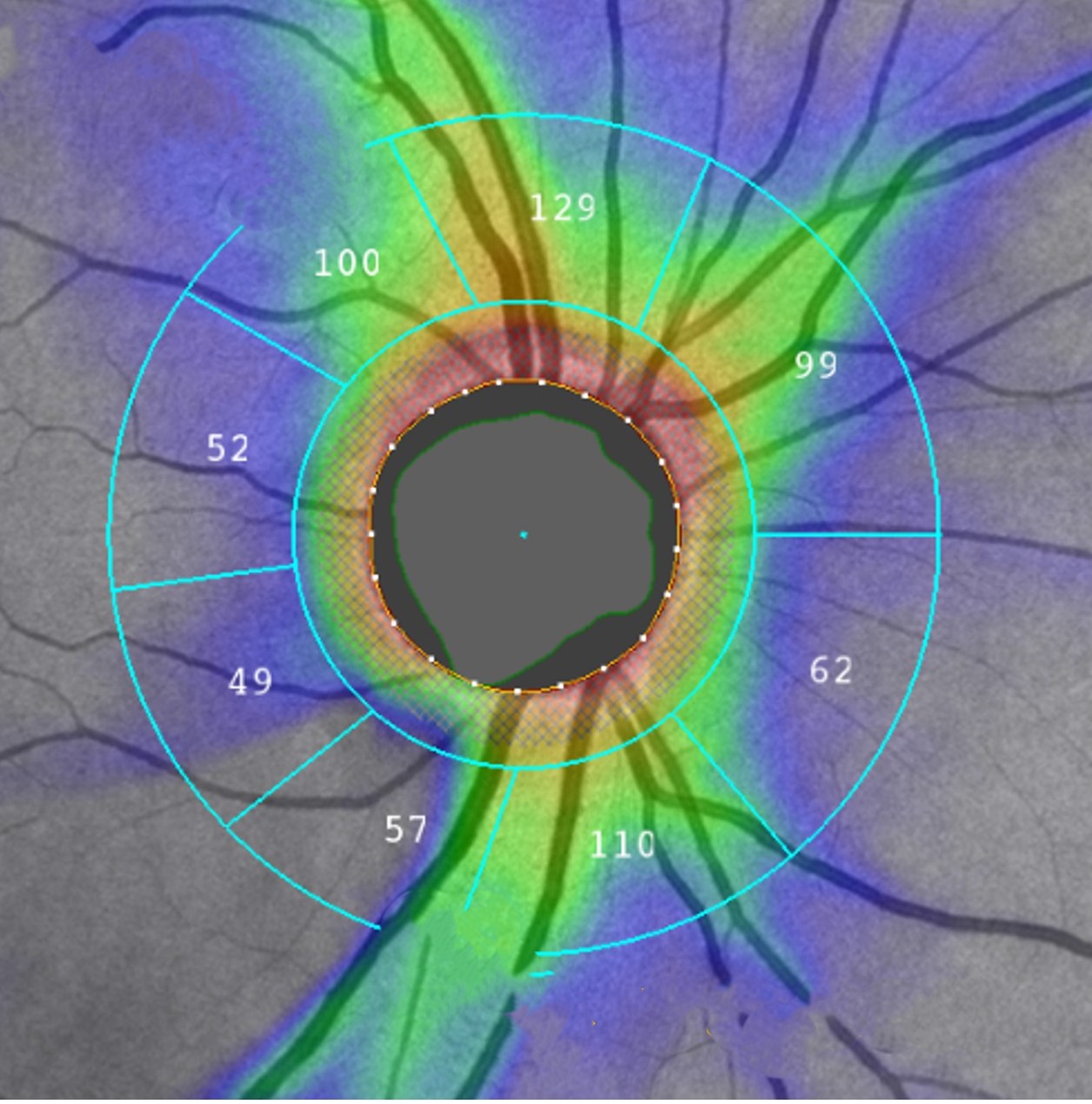
Today, the most widely used and trusted imaging tool for diagnosing glaucoma is optical coherence tomography. In routine clinical practice, doctors rely primarily on retinal nerve fiber layer thickness measurements from OCT scans to detect and monitor glaucomatous damage. Typically, the NFL thickness profile is measured along a circle around the optic nerve head, where the nerve fibers exit the eye and carry visual information to the brain.
OCT can measure the NFL’s thickness profile with micrometer-level precision. However, despite its widespread use, this profile alone is often not sensitive enough to detect early glaucoma. This is because individual NFL thickness varies widely in normal eyes.
“The way glaucoma is diagnosed today with OCT is based on comparing an individual eye’s NFL thickness profile with the normal range,” explains Tan. When doctors compare a patient’s NFL thickness profile to a broad range, small but meaningful early damage may not be evident.
Generative artificial intelligence helps doctors interpret medical tests better by learning what is normal for each patient. By analyzing complex information—such as age, sex, height, weight and clinical history—AI can narrow down the range of normal test results for an individual patient and detect subtle changes that might otherwise go unnoticed. It can also analyze patterns from multiple tests and clinical information sources to suggest likely diagnoses. The result is better-informed decision making and care that is better tailored to each patient.
Tan’s team applies this generative AI technology to OCT imaging to estimate an individualized baseline of NFL thickness for each eye. In other words, instead of comparing a patient to a broad range of other people, the AI predicts what that specific eye’s normal NFL thickness should be, based on its unique characteristics. Once this personalized baseline is established, relatively minor changes—often missed by standard methods—become easier to detect.
Earlier approaches to personalization focused on age, sex, and race. While helpful, these factors alone do not explain most of the variability seen in real patients. Tan’s innovative approach goes beyond basic demographics to further incorporate the eye’s size, shape and retinal vascular patterns, all derived directly from the same OCT scan. No additional imaging techniques, tests or equipment are required.
“We designed this to be simple and practical,” says Tan. “Everything comes from a single OCT scan that patients already receive in routine eye care.”
By personalizing NFL thickness measurements, this AI-based approach can reduce false alarms, especially in people with unusual eye anatomy, while at the same time improving sensitivity to the earliest signs of glaucoma—when treatment is most effective at preserving vision.
For patients, this means a better chance of detecting glaucoma before permanent damage occurs. For clinicians, it means greater confidence in early diagnosis. And for society, it offers a scalable, accessible path toward earlier and more personalized glaucoma care worldwide.
“Our goal is to help everyone benefit from earlier glaucoma detection,” Tan says. “By personalizing the most widely used OCT measurement with AI, we can protect vision sooner and more effectively.”
Dr. Ishikawa: Forecasting the future to guide treatment decisions
Dr. Hiroshi Ishikawa, professor of ophthalmology at OHSU, uses AI to predict what will happen next. "With AI, we're forecasting what the patient's glaucoma status will be on the next visit."
Glaucoma progresses slowly and causes irreversible damage, making early intervention critical. "The big win is stopping progression and maintaining the vision people have," Dr. Ishikawa explains.
The challenge? Visual field testing is subjective and suffers from high variability. "The input is completely dependent on the patient pressing a button," Dr. Ishikawa says. "It's also boring, so patients often fall asleep during the test." Patients need five or six tests over two to three years before clinicians can confidently identify progression patterns.
"We don't want to wait two or three years," Dr. Ishikawa says. "That's where AI comes in. One test, or maybe two—just a six-month gap—using AI can tell you if this patient's disease will progress or not."
Dr. Ishikawa's team developed AI models that forecast disease progression after just one or two visits, giving clinicians actionable information much earlier. "If the accuracy of forecasting is good enough, the physician can decide at the first or second visit what to do," he explains. "The patient may need more medication or even surgery, depending on progression speed."
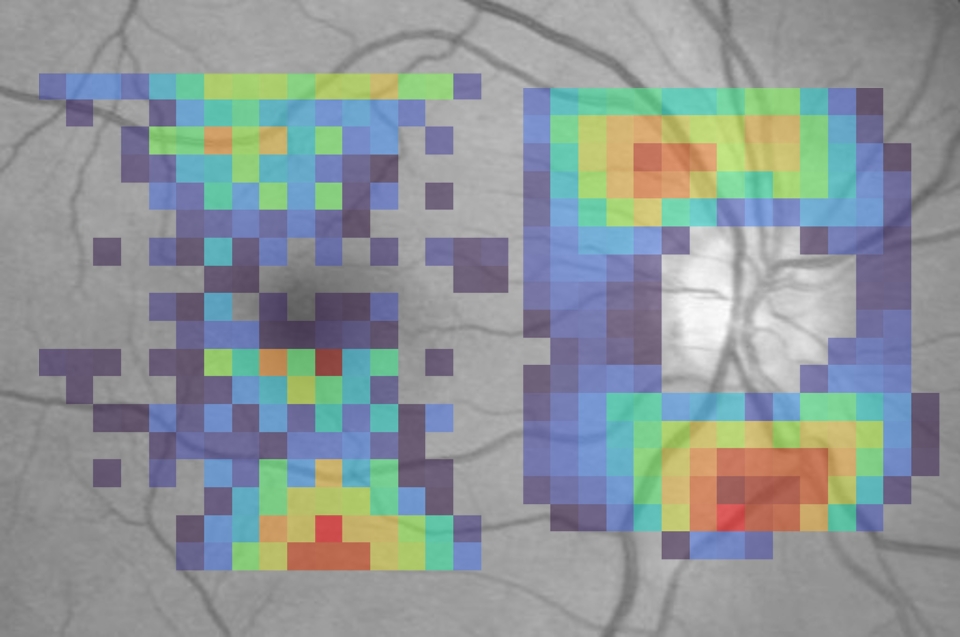
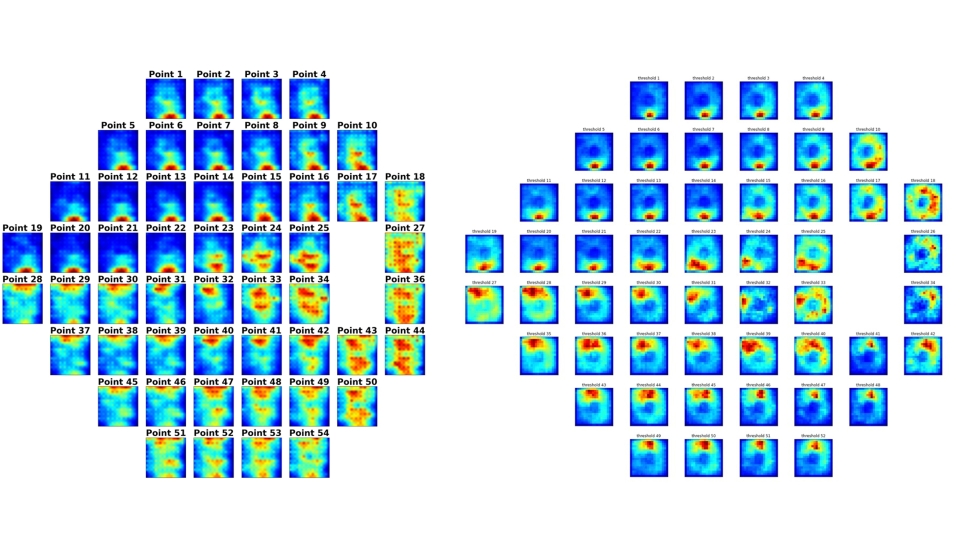
Dr. Ishikawa's research also leverages OCT imaging. "In a single second, we get a three-dimensional image, like an MRI of the retina," he says. His team developed AI that estimates visual field results from these structural OCT scans—invaluable when patients miss appointments or skip the tedious test. "OCT-generated visual field testing can function as a substitute for actual visual field testing."
Dr. Ishikawa also employs attention analysis techniques that reveal which regions of an OCT image the AI examines to generate its predictions. "We can generate a heat map," he explains. Traditional mapping divides the visual field into six sectors. "AI gives us a high-definition version of structure-function mapping."
Dr. Ishikawa's work draws on extensive databases from his collaboration with Joel Schuman, M.D., at Wills Eye Hospital, where many patients have more than twenty years of longitudinal data. He is now extending this research at Casey Eye Institute, collaborating with colleagues including Aiyin Chen, M.D., and Michael Gale, M.D. In the future, his group could create software to make their forecasting system clinic ready.
Team science for complex challenges
Both researchers emphasize that AI-driven glaucoma research requires collaboration. Tan builds comprehensive datasets from Casey Eye Institute's busy clinic, public databases and government-funded projects. Dr. Ishikawa partners with institutions across the country, leveraging decades of patient data.
These complementary research programs demonstrate Casey Eye Institute's leadership in applying artificial intelligence to tackle sight-threatening diseases. Tan and Ishikawa join colleagues like Aiyin Chen, M.D., and Alireza Karimi, Ph.D., who are also pioneering AI applications in glaucoma research at Casey.
Both approaches share a common goal: detecting disease progression earlier and more accurately, enabling timely intervention to preserve vision. As these AI tools move from research to clinical practice, they promise to transform glaucoma care for patients worldwide.