Lipid Nanoparticles Offer Promising New Approach
In the rapidly evolving gene therapy landscape for ophthalmic genetics, Casey Eye Institute researcher Renee Ryals, Ph.D., is pioneering a new approach to inherited retinal diseases. Her lab, in collaboration with Gaurav Sahay, Ph.D. at Oregon State University, is focusing on the development of lipid nanoparticle (LNP) delivery systems—a technology that promises to overcome challenges and expand therapeutics options for genetic eye disease treatment.
From viral vectors to mRNA
Most eye care professionals and researchers understand the frustration that follows a diagnosis of inherited retinal disease (IRD). To date, more than 250 genes are linked to these conditions, but only one IRD has an approved gene therapy (voretigene neparvovec, approved in 2017 to treat Leber congenital amaurosis and sold under the brand name Luxturna).
Casey researcher Mark Pennesi, M.D., Ph.D., and his colleagues helped gain approval from the US Food and Drug Administration for this treatment. With support from Editas Medicine, Dr. Pennesi also led the first Phase 1/2 clinical trial evaluating an in vivo CRISPR/Cas9 gene editing technology for inherited retinal degeneration.
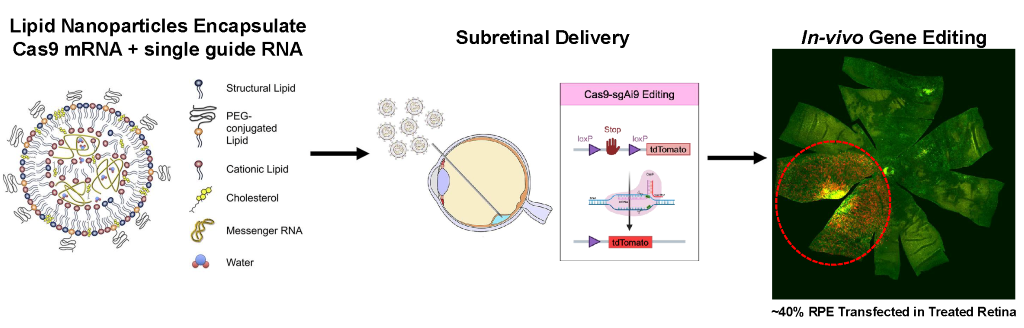
Now the Ryals Lab is building on Casey’s legacy of research into genetic and eye diseases. While current gene therapies often rely on AAV vectors, these have some limitations in terms of cargo capacity. The Ryals Lab is exploring ways to address these challenges with the aim of developing safer and more effective gene therapies. In gene therapy with LNPs, the nanoparticles deliver messenger RNA (mRNA) to the treatment area. This offers unique advantages, particularly for gene editing applications.
"The benefit of using mRNA is that the protein degrades and doesn't persist long-term," Dr. Ryals explains. "This makes it potentially ideal for gene editing, where we can deliver the editor to photoreceptors and make a permanent edit to the host genome, but then the editor itself naturally degrades, leaving behind only the beneficial genetic correction."
This work began before the COVID-19 pandemic brought LNPs into the spotlight. "The field has really boomed since the COVID-19 vaccines, which use the same basic delivery vehicle,” says Dr. Ryals. “We're taking that conventional LNP platform and modifying it specifically for the retina."
Key advantages of LNPs
At the heart of Dr. Ryals's work is the elegant engineering of LNPs. These nanoparticles are composed of four carefully selected components: an ionizable lipid, cholesterol, a structural lipid, and a PEG lipid. Through microfluidic mixing with nucleic acids, these particles develop a distinctive core and shell architecture that enables efficient cell internalization and targeting.
Although the Ryals lab is focused on understanding how these LNPs get internalized into the photoreceptors, her group is exploring all the potential targets in the eye. “By modifying the lipids, lipid ratios or adding peptides, we can target many different retinal cells," says Dr. Ryals. ““Additionally, by changing the injection routes, different tissues become accessible.” The team is using intravitreal injections to reach ganglion cells and intracameral delivery to access the trabecular meshwork for potential glaucoma treatments.
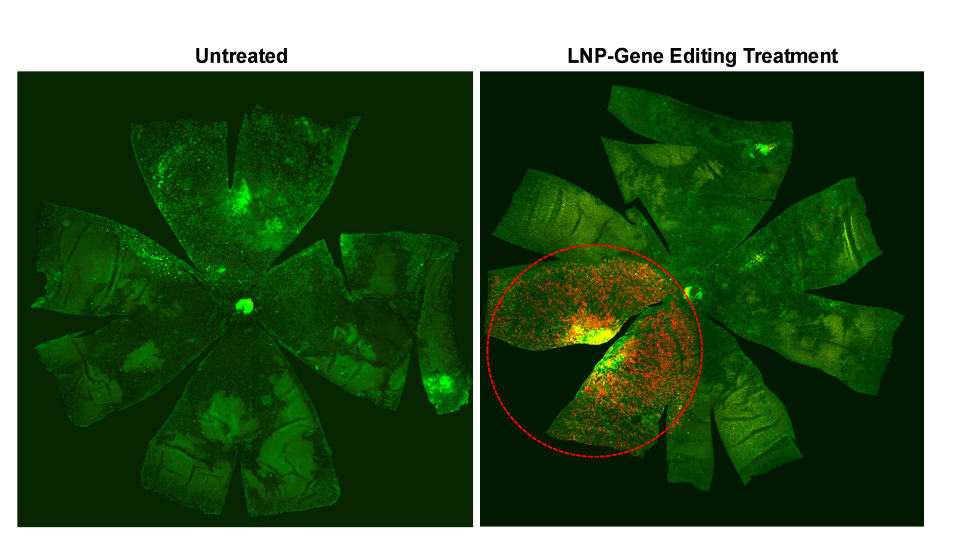
Pioneering LNP delivery in retinal research
The Ryals Lab stands out as one of the first research groups to demonstrate successful LNP delivery in both mice and nonhuman primates. The team has achieved significant success in delivering gene editors to the retinal pigment epithelium (RPE), achieving approximately 40 percent transfection efficiency in treated areas.
Dr. Ryals' team is at the forefront of innovation, leveraging members’ expertise to develop groundbreaking lipids and peptides that overcome critical delivery challenges in gene therapy. By integrating these advancements with the resources of the Casey Eye Institute Retinal Stem Cell Center, they are poised to accelerate the development of transformative treatments for retinal diseases.
Building a pipeline to treatment
Today, Casey researchers conduct more gene therapy clinical trials than almost any location worldwide. Working with the Retinal Stem Cell Center, directed by Kathleen Chirco, Ph.D., makes it possible for the Ryals team to work with banked patient cells, design gene editing machinery, test delivery vehicles, and evaluate effectiveness using next-generation sequencing.
"We are developing a pipeline where we can take a patient sample, design and evaluate the mutation-specific gene editing machinery, ensure we have effective delivery vehicles , and test the therapeutic product," says Dr. Ryals. This approach could accelerate the development of personalized treatments for inherited retinal diseases.
Looking to the future
Supported by a $3.1 million grant from the National Eye Institute, Dr. Ryals continues to refine this technology. Current research focuses on achieving efficient gene editing in photoreceptors, showing the translation of gene editing in the retina from mouse to non-human primates and exploring different delivery routes including intravitreal, suprachoroidal, and intracameral.
The LNP platform’s flexibility allows for various therapeutic approaches, including gene augmentation, knockdown or editing. The technology shows particular promise for inherited retinal disease and glaucoma treatment.
As the field of mRNA therapeutics continues to gain recognition, including recent Nobel Prize-winning work, the Ryals Lab's pioneering research in ocular applications positions them at the forefront of developing next-generation treatments for devastating eye diseases. For patients who have previously had limited options, this could mean potential prevention of vision loss, more targeted genetic interventions, and hope for conditions once considered untreatable.