Family Planning Program Research
Current research efforts
Cochrane Fertility Regulation Review Group (Cochrane FRG)
The Cochrane FRG, the only full Cochrane review group based in the continental US, is dedicated to producing high impact systematic reviews focused on topics related to contraception and abortion.
Oregon National Primate Research Center (ONPRC)
The ONPRC is one of seven National Primate Research Centers supported by the National Institutes of Health to pursue basic and translational research in order to help people across generations live longer, healthier lives through scientific breakthrough. Our clinician scientists work closely with the ONPRC and several hold a joint faculty position with the ONPRC’s Division of Reproductive and Developmental Sciences, led by Dr. Jon Hennebold.
Oregon Permanent Contraception Research Center (OPERM)
OPERM provides grant funding, scientific expertise, a nonhuman primate (NHP) animal resource, laboratory and procedural infrastructure, and administrative support to investigators who wish to evaluate novel agents or procedures for permanent female contraception.
Women’s Health Research Unit (WHRU)
The WHRU is the Department of OB/GYN’s clinical trials unit. Originally launched by Dr. Leon Speroff, one of the ‘Giants’ in the field of OB/GYN, the WHRU contains the physical infrastructure and experienced staff to manage and support investigators with investigator initiated studies, trials for industry, and foundation sponsors.
Nonsurgical permanent contraception for women. Expanding access and reducing cost (PI Jensen)
Women who have completed desired family size and seek permanent contraception must undergo invasive surgical procedures. In 2014, with generous support from the Bill & Melinda Gates Foundation, we established the Oregon Permanent Contraception Research Center (OPERM) at the ONPRC to address the unmet needs of these women through the development of a simple, low-cost, safe, and highly effective method of nonsurgical permanent contraception (NSPC). We renewed OPERM in 2018, with a focus on moving a lead method into clinical trials. OPERM provides a research center with scientific expertise, a nonhuman primate (NHP) animal resource, laboratory and procedural infrastructure, and administrative support to investigators who wish to evaluate novel agents or procedures for permanent female contraception. Over the last 6 years, we have evaluated a number of approaches to NSPC. We have identified a lead approach of polidocanol foam (PF), and several promising alternative methodologies. We have developed a collaboration with the Population Council to complete GLP (Good Laboratory Practices) toxicology studies and GMP (Good Manufacturing Practices) formulation development studies and other regulatory requirements needed to support an FDA Investigational New Drug (IND) submission. However, since we recognize and accept the inherent risks associated with clinical product development, we continue our preclinical studies evaluating alternative approaches to NSPC using the guinea pig and baboon model at the Oregon National Primate Research Center concurrent with the PDF program. The ultimate goal of this research program is the development of a low-cost highly effective nonsurgical method of permanent contraception for women with an appropriate delivery technology for low resource settings. Such a product would also meet the needs of women seeking permanent contraception in high resource nations, by increasing convenience and safety. An external Scientific Advisory Board (SAB) provides oversight for both the IND-enabling activities and for our research and development program for alternative methodologies including scientific review of proposed experiments, guidance on “go/”no go” decisions and the prioritization of resources. In addition to new agents, we plan to develop novel delivery methodologies to control intra uterine/fallopian administration of agents. Funded by the Bill & Melinda Gates Foundation.
Publications (2019-2020):
-
Patil E, Thurmond A, Hart K, Seguin J, Edelman A, Jensen JT. Intrauterine fluid instillation to confirm tubal occlusion after transcervical permanent contraception: A pilot study(). Contraception. 2020;101(1):40-5.
-
Jensen JT, Hanna CB, Yao S, Mishler E, Chai D, Kiulia NM, et al. Polidocanol/doxycycline foam for nonsurgical permanent female contraception: 6 month data baboon contraception study. Fertility and Sterility. 2019;112(3):e305-e6.
Nonhormonal on-demand contraception for women (PI Jensen)
An orally active, female-controlled agent that prevents maturation or fertilization of the oocyte without affecting menstrual cycles would be a game-changing strategy for contraception. Such an approach would prove useful for regular daily use and greatly extend the time range of effectiveness of on-demand contraception. Recently, whole exome sequencing approaches evaluating otherwise healthy women with infertility due to meiosis and fertilization failure have validated several oocyte-specific protein as potential targets. Unfortunately, efforts to develop classical inhibitors have not succeeded for several reasons, including highly conserved regions around the enzyme-binding site. We currently lack alternative approaches to classical inhibitors, specific and efficient high-throughput methods for screening compound libraries for activity, and appropriate biologic testing methods short of fertility studies to screen lead compounds. Generous funding from the Bill &Melinda Gates Foundation supported work in our olab in collaboration with the Institute of Drug Development at the University of Minnesota to identify and test novel drugs to inhibit or block key proteins involved in oocyte maturation. Our lead target is WEE2 kinase, a specific regulator of meiosis. The nonhuman primate model at ONPRC allows us to evaluate the proof of concept of the effectiveness of selected agents to block meiosis and fertilization without affecting menstrual cycle regularity. Funded by the Bill & Melinda Gates Foundation.
Publications (2019-2020):
- Hanna CB, Mudaliar D, John K, Allen CL, Sun L, Hawkinson JE, et al. Development of WEE2 kinase inhibitors as novel non-hormonal female contraceptives that target meiosisdagger. Biol Reprod. 2020;103(2):368-77.
- Hanna CB, Yao S, Martin M, Schönbrunn E, Georg GI, Jensen JT, et al. Identification and Screening of Selective WEE2 Inhibitors to Develop Non-Hormonal Contraceptives that Specifically Target Meiosis. ChemistrySelect. 2019;4(45):13363-9.
- Alton K, Jensen J. Update on Permanent Contraception for Women. Current Obstetrics and Gynecology Reports. 2018;7(4):163-71.
Early pregnancy evaluation: Diagnosis and outcomes in pregnancy of unknown location (PI Baldwin)
One-quarter of all pregnancy-related deaths from massive bleeding are from undiagnosed ectopic pregnancies, where embryo implantation occurs outside the uterus. Clinicians struggle with prompt and accurate diagnosis of these abnormal pregnancies, as they are initially indistinguishable from normal pregnancy. No significant advancements in clinical diagnosis of ectopic pregnancy have occurred over the last two decades. Currently, there is no reliable, non-invasive diagnostic test to confirm uterine location of the pregnancy until two weeks after implantation with ultrasonography. The goal of this work is to develop a minimally invasive test to diagnose ectopic pregnancies before they lead to poor outcomes, and to do this in a way that reduces patient testing,worry, and unnecessary interventions. Researchers have attempted to identify markers of early pregnancy in blood tests that might distinguish between uterine or tubal location, but they have not been able to distinguish between abnormally-growing uterine pregnancy and ectopic implantation. Our work thus far has suggested that samples taken in closer proximity to the implantation site will improve specificity compared to serum measurements. With funding from the NIH K12 Women’s Reproductive Health Scholar Program, Dr. Baldwin is currently evaluating the diagnostic capability of genetic biomarkers from non-invasive samples.
Oxytocin Receptor Expression in Pregnancy (PI Reid)
Obstetric hemorrhage is the leading cause of maternal mortality worldwide and is also the leading cause of maternal mortality after interruption of pregnancy in the second trimester. Studies evaluating the management of hemorrhage after termination in the second trimester are limited and do not provide clear physiologic evidence to guide effective prevention or treatment strategies. Oxytocin is the most effective pharmacologic agent for prophylaxis of postpartum hemorrhage after a full-term delivery, but its utility in the second trimester is unknown. Prior research has indicated that there is low oxytocin receptor expression in the myometrium in early pregnancy, with higher expression at full-term and with the onset of labor. However, we do not know when during gestation oxytocin receptor expression increases significantly above nonpregnant levels, nor when the myometrium becomes responsive to oxytocin. This lack of knowledge about oxytocin receptor expression has led to inconsistent practice in the prevention and management of post-abortion hemorrhage – some physicians use exogenous oxytocin at gestational ages when it may be ineffective and others forego oxytocin when there may be a benefit. This project examines oxytocin receptor expression by gestational age to provide basic science guidance to this clinical question. This work is funded through a Society of Family Planning Research Fund Grant.
Abortion in Mexico: Responding to advocates’ need for evidence (PI Darney)
This decade-long body of work has mutually beneficial collaboration and capacity-building for research at its core. We leverage medical charts, discharge data, and survey data to answer key questions around the public abortion program in Mexico City, public opinion on abortion, and utilization of abortion-related services across Mexico. This program of research is in collaboration with the Reproductive Health program of the Ministry of Health of Mexico City, Ipas, Catholics for Free Choice (Catolicas por el derecho a decider/CDD), ECOSUR (el colegio de la frontera sur, San Cristobal, Chiapas), The Population Council, and CISIDAT (a non-profit health research consortium). Our team prioritizes training and career development; junior researchers from the US and Mexico actively participate in the team, leading analyses and scientific publications. Our work has examined who presents late for legal abortion and cannot receive services, gestational age estimation using LMP and U/S, disparities in access to legal abortion in the greater Mexico City metropolitan area, implementation of legal exceptions, measurement of quality of care, availability of misoprostol in pharmacies, use of second trimester services, out of facility abortion models, and case-fatality due to abortion nationwide. Our work speaks to current political rhetoric around, for example, whether abortion rates continue to rise after legalization, whether Mexico’s Catholic population supports access to abortion and under which circumstances, whether Mexican women need second trimester abortion services, and disparities in access to basic healthcare. A bibliography is attached.
Our program of research on access to contraception in Mexico focuses on who (e.g. adolescents) receives contraception, where (e.g. post-partum vs. clinic setting) services are delivered, and the quality of those services. We identify disparities in access and gaps in service delivery that inform policy debates about how to address Mexico’s high rates of adolescent births.
Publications:
- Early termination of pregnancy: differences in gestational age estimation using last menstrual period and ultrasound in Mexico.
Saavedra-Avendano B, Schiavon R, Sanhueza P, Rios-Polanco R, Garcia-Martinez L, Darney BG.Reprod Health. 2020 Jun 9;17(1):89. - Darney BG, Fuentes-Rivera E, Polo G, Saavedra-Avendaño B, Alexander LT, Schiavon R. Con la ley y sin la ley/With and without the law: Utilization of abortion services and case fatality in Mexico, 2000-2016. Int J Gynaecol Obstet. 2020 Mar;148(3):369-374. doi: 10.1002/ijgo.13077. Epub 2019 Dec 27. PubMed PMID: 31821537; PubMed Central PMCID: PMC7027437.
- Weaver G, Schiavon R, Collado ME, Küng S, Darney BG. Misoprostol knowledge and distribution in Mexico City after the change in abortion law: a survey of pharmacy staff. BMJ Sex Reprod Health. 2019 Nov 5;. doi: 10.1136/bmjsrh-2019-200394. [Epub ahead of print] PubMed PMID: 31690579; PubMed Central PMCID: PMC6978560.
Examining the role of incentive measures in improving effective contraceptive use (PI Rodriguez)
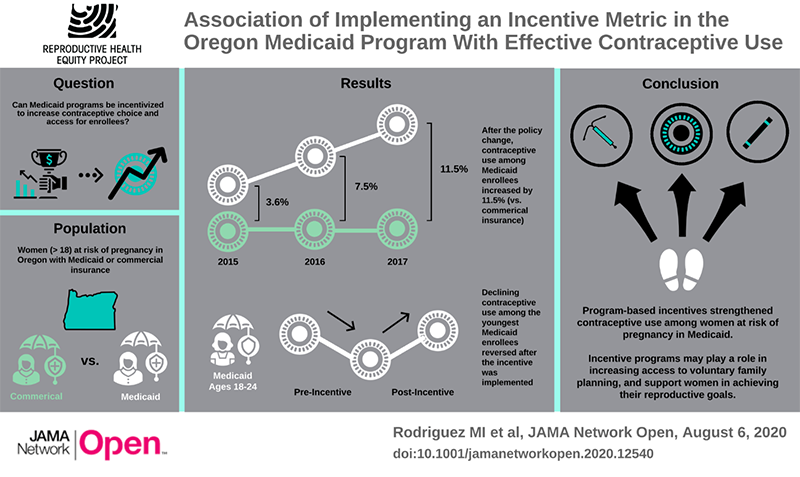
Oregon was the first state to implement a financial incentive for providing effective contraception within their Medicaid program. Oregon’s Effective Contraceptive Use (ECU) metric assesses the percentage of reproductive age women at risk for unintended pregnancy who are provided with an effective contraceptive method. The ECU metric is one of a pool of 17 quality metrics, and Coordinated Care Organizations (CCOs) who meet state goals for these metrics, receive a financial incentive. The state goal for the ECU metric is 50% of all women, or an improvement from the prior year’s score. This is not an incentive to women or providers, which could lead to reproductive coercion, but an incentive at the program level.
A similar metric has recently been endorsed by the federal Office of Population Affairs as a quality indicator for national use, however it is not nationally implemented. It is important to study the impact of financial incentives on contraception to identify how they impact contraceptive initiation, continuation and unintended pregnancy rates while promoting reproductive autonomy.
This comparative interrupted time series study found that a program level incentive was associated with an increase in the use of effective contraception among women enrolled in Medicaid (the target of the metric was associated with reversal of a declining trend in contraceptive use among young women.
Publications:
- Rodriguez MI, Meath T, Huang J, Darney BG, McConnell KJ. Effective Contraceptive Use After Implementation of an Incentive Metric in Oregon's Medicaid Program. JAMA Network Open, August 6, 2020.
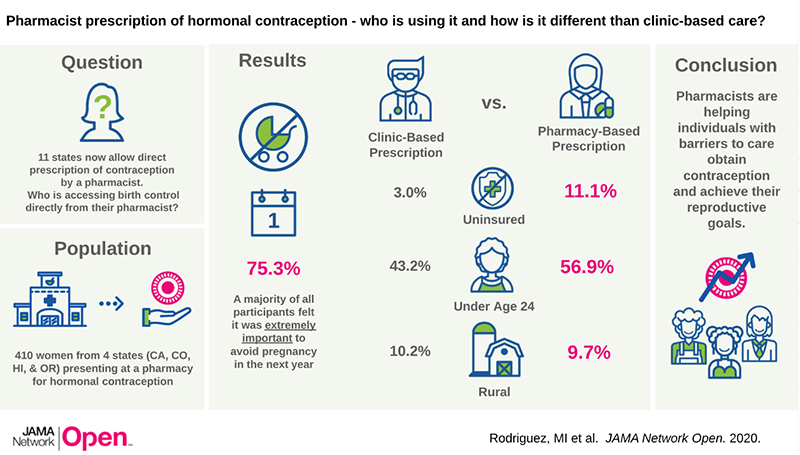
Pharmacists Expand Access to Reproductive heaLthcare: the PEARL study (PI Rodriguez)
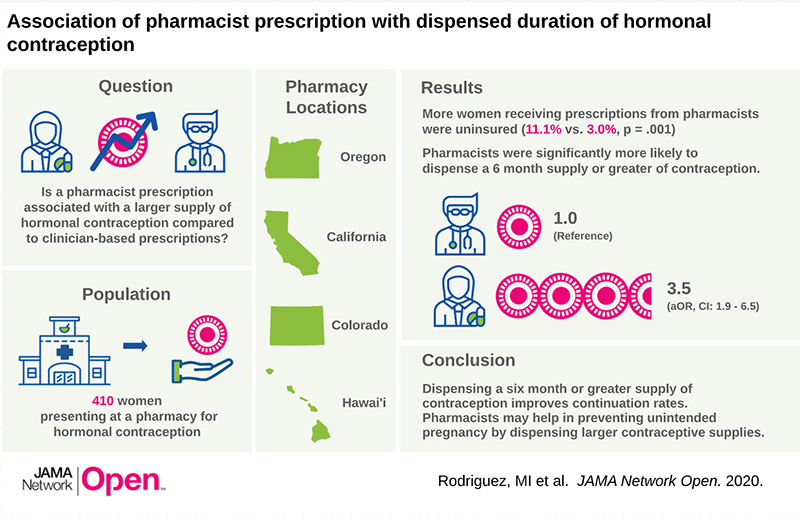
In passing House Bill 2879 in 2016, Oregon became the first of now many states to allow pharmacists to directly prescribe hormonal contraception (“HC”), including the pill, patch, or ring, without a clinic visit.8 Two years following passage of this policy, the majority of retail pharmacists across the state of Oregon were certified by the Board of Pharmacy to prescribe HC (Rodriguez et al, Journal of the American Pharmacist Association, August 2018). Our research has national relevance: since Oregon’s policy was implemented, elven other states have begun pharmacist prescription of HC, and more are beginning the process.
We utilized two distinct and complementary methodologies to evaluate the effect of pharmacist prescribing policy on key outcomes including women’s satisfaction and acceptability of care, contraceptive continuation, and quality of care. We conducted a prospective clinical cohort study to directly measure the impact on contraceptive continuation, rates of unintended pregnancy, and to understand who is accessing contraceptive care in pharmacies. We recruited women from four states (CA, CO, HI and OR) and have demonstrated that pharmacists are more likely to prescribe a larger supply of contraceptives, and to reach uninsured and younger women. These findings suggest that pharmacists have an important role to play in reducing unintended pregnancy and associated public costs. Importantly, pharmacists in rural areas, are as likely as their urban peers to prescribe contraception, which may improve access for women with geographical barriers to care. We are currently completing one year follow up with our cohort to determine the association of pharmacist prescription of contraception with method continuation. We are also conducting a database study to examine the quality and safety of care, as well as the association with contraceptive breaks.
Publications:
- Rodriguez MI, Anderson L, Edelman AB. Pharmacists begin prescribing hormonal contraception in Oregon: Implementation of House Bill 2879. Obstetrics and Gynecology July 2016.
- Rodriguez MI, Hersh A, Anderson L, Hartung DM, Edelman AB. Association of Pharmacist Prescription of Hormonal Contraception with Unintended Pregnancy and Medicaid Costs. Obstetrics & Gynecology, June 2019.
- Rodriguez MI, Garg B, Williams S, Souphanavong J, Schrote K, Darney BG. Availability of pharmacist prescription of contraception in rural areas of Oregon and New Mexico. Contraception, e-published December 2019.
- Rodriguez MI, Edelman AB, Skye M, Anderson L, Darney BG. Association of pharmacist prescription and dispensed duration of hormonal contraception. JAMA Network Open. 2020;3(5):e205252. doi:10.1001/jamanetworkopen.2020.5252 (Reprinted)
Medicaid policy and reproductive health inequities: immigrant health (PI Rodriguez)
Comprehensive reproductive health care is essential to the health and well-being of individuals and their communities. Despite gains in coverage and health made with Medicaid expansion, a key population remains systematically excluded from care: new and undocumented immigrants.
Emergency Medicaid is a safety net program that covers individuals who meet the financial requirements for traditional Medicaid, but not the citizenship requirements. Emergency Medicaid covers only acute life-threatening events and obstetric admissions. For example, federal Emergency Medicaid covers the cost of a delivery, but provides no funds for prenatal care or post-partum contraception. States can choose to use their own funds to expand coverage for the immigrant population. Our portfolio of work examines how Medicaid restrictions to health care based on citizenship influence reproductive health disparities and Medicaid costs.
Publications:
- Rodriguez MI, Jensen JT, Darney PD, Little SE, Caughey AB. “The Financial Impact of Extending Postpartum Contraception to New Immigrants.” Obstetrics and Gynecology, March 2010.
- Rodriguez MI, Caughey AB, Edelman AB, Darney PD, Foster DG. “Cost-benefit analysis of state and hospital funded postpartum intrauterine contraception for new immigrants at a university hospital.” Contraception, April 2010.
- Swartz J, Hainmueller J, Lawrence D, Rodriguez MI. “Expanding prenatal care to immigrant women reduces infant mortality.” Obstetrics and Gynecology, October 2017
- Rodriguez MI, Garg B, Funkhouser S, Swartz JJ, Baldwin MK. Immediate postpartum long acting, reversible contraceptive use among the Emergency Medicaid population. AJOG, e-published December 2019.
- Rodriguez MI, Swartz JJ, Hainmuller J, Lawrence D, Caughey AB. Extending prenatal care to unauthorized immigrants improves health outcomes and reduces Medicaid costs
- Accepted to Women’s Health Issues, February 2020
Contraceptive services for young women in the US safety net 2010-2016: the role of Title X and school-based clinics (PI Darney)
Unintended pregnancy is endemic in the United States. We will generate evidence about the roles of Title X and school based clinics in determining access to and quality of contraceptive services in the US safety net. This proposal moves beyond documenting disparities by ethnicity to identify how multilevel factors influence access, quality, utilization, and discontinuation and drive differences in effective contraception use and ultimately unintended pregnancy. Understanding these relationships addresses two key OPA priorities: Priority 1, Title X current services and changes over time, and Priority 3, service delivery innovations: school-based clinics. Our unique dataset enables us to integrate new OPA quality metrics with electronic health record (EHR) data linked with clinic level, county-level social determinants of health, and state level policies to examine factors related to access and utilization of contraception.
¡SALE!: Social determinants of health and Adolescent pregnancy in Latinas (PI Darney)
Latina adolescents in the US have over twice the adolescent birthrate as non-Hispanic white adolescents. Furthermore, unintended pregnancy is increasingly concentrated in non-white populations, among younger ages and poor women, and at the intersection of these individual socio-demographic factors. This proposal includes innovative analyses of existing data, new and advanced methods of analysis, and novel integration of datasets to allow to exploration of new questions in adolescent pregnancy and reproductive behaviors and outcomes in a key population that experiences high rates of adolescent and unintended pregnancy, Latinas. We will use a socio-ecological framework to guide our binational, multidisciplinary team and multi-level analysis to assess social determinants of health - the roles of immigration and community level vulnerability - and adolescent pregnancy and additional reproductive outcomes. We will leverage binational data that permit us to construct innovative comparison groups. Results from this study will allow pregnancy prevention programs to better integrate a targeted socio-ecological approach as part of evidence-based pregnancy prevention interventions. Integrating social determinants of health into interventions has the potential to decrease the stark disparities adolescent pregnancy and birthrates among Latinas in the US and improve educational, economic, and health outcomes for all women.
Management of Cesarean Scar Ectopic Pregnancy and Cervical Ectopic Pregnancy (PI Reid)
Ectopic pregnancies pose significant risk to women’s health. While 90% of ectopic pregnancies occur within the fallopian tube, ectopic pregnancies may implant in other sites within the abdomen and pelvis. Evidence-based guidelines exist regarding the management of tubal ectopic pregnancies. In contrast, non-tubal ectopic pregnancies are rare and quality evidence is limited concerning the diagnosis and management of non-tubal ectopic pregnancies. Clear guidelines do not exist and clinical practice is variable by institution and physician experience. This project creates a prospective multi-site repository with the goal of determining evidence-based guidelines for the management of cesarean scar ectopic and cervical ectopic pregnancies.
Contraceptives for Men (PI Edelman) – Study currently enrolling
Preventing or planning pregnancy takes two and empowering men through the development of new contraceptive options helps to improve everyone’s ability to be involved in their family planning goals. In conjunction with the National Institutes of Health Contraceptive Clinical Trials Network for Male Contraceptive Development, we are investigating a hormonal contraceptive skin gel that temporarily suppresses sperm production. This gel is placed on the skin daily and absorbed through the skin like a lotion. This study is enrolling ‘couples’ as we are investigating how well the gel prevents pregnancy. We monitor the male partner’s sperm count regularly to ensure that it is in the correct range. If you or your partner might be interested, see https://www.ohsu.edu/womens-health/male-contraception-study
Finding solutions to problematic bleeding in contraceptive implant users (PI Edelman)
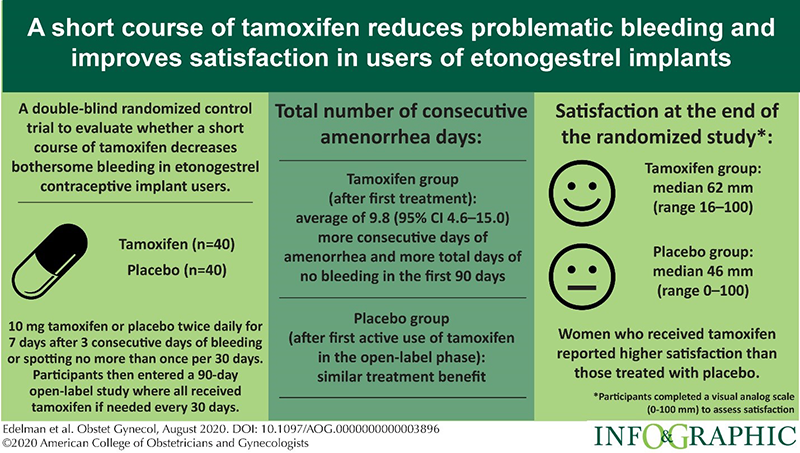
Contraceptive implants are highly effective methods of contraception but some users experience changes in their bleeding pattern that is unacceptable. Clinicians lack treatment options for this problematic bleeding which leads many users to be dissatisfied and stop using the method. We found that a short course of tamoxifen (7 days) helps to temporarily stop bleeding and provide those using tamoxifen, more days of no bleeding and more days of lighter bleeding (e.g. spotting). This data and our prior study support the use of tamoxifen as an effective and well-tolerated option to treat problematic bleeding in implant users. We have ongoing studies to test other potential treatment options.
References:
- Simmons KB, Edelman AB, Fu R, Jensen JT. Tamoxifen for the btreatment of breakthrough bleeding with the etonogestrel implant: a randomized controlled trial. Contraception 2017; 95:198–204.
- Edelman AB, Kaneshiro B, Simmons K, Hauschildt J, Bond K, Boniface E, Jensen JT. Treatment of Unfavorable Bleeding Patterns in Contraceptive Implant Users. Obstet Gynecol 2020; 136: 323-332.