Doris Kretzschmar Lab

Eve Lowenstein, Alex Law (Aug 2022)
Research interests
The focus of Dr. Kretzschmar’s work is to identify genetic factors and mechanisms that lead to progressive degeneration of the adult nervous system using Drosophila as a model for human diseases.
Current projects
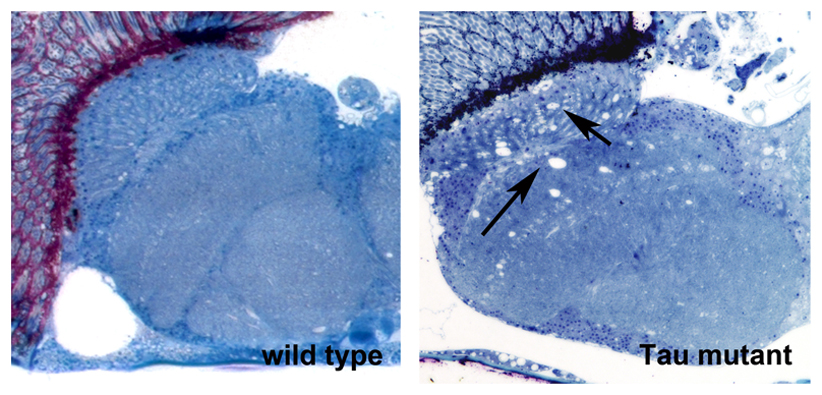
We are using Drosophila to study basic mechanisms of neurodegeneration. Currently, we are focusing on two proteins that play key roles in Alzheimer’s disease; the Amyloid Precursor Protein and Tau. We are investigating what effects disease-associated changes in these proteins have on their functions and how this effects the genetic pathways these proteins are involved in. To address this, we use behavioral assays, histology and confocal imaging, as well as various molecular and sequencing techniques. Using genetic manipulations, we study whether other proteins can ameliorate disease-associated phenotypes or contribute to the pathogenicity. In a second project, we are investigating effects of aging on behavior and the function of the nervous system. In addition, my group is part of a research center that tests effects of botanical dietary supplements on enhancing resilience during aging and in neurodegenerative diseases.
Postdoctoral researcher

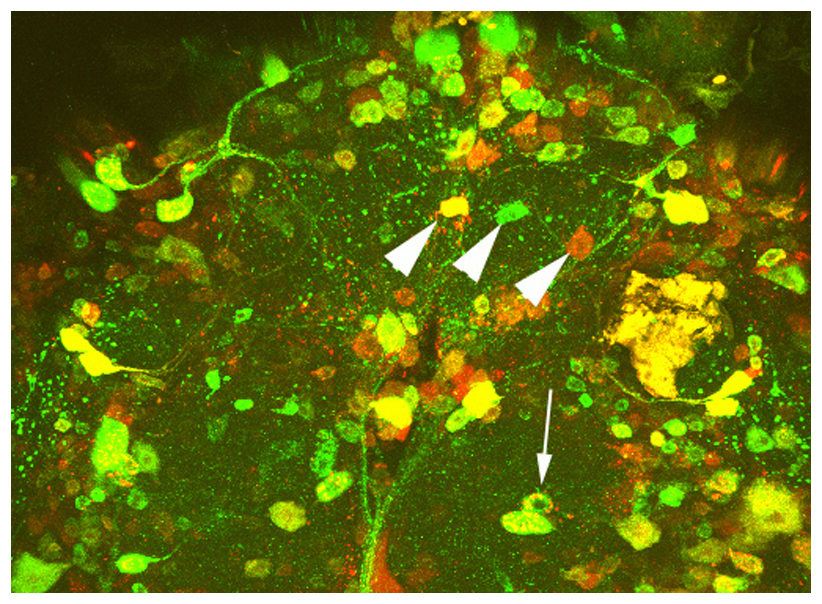
Sleep and other circadian disruptions occur during normal aging but are also a hallmark of several neurodegenerative diseases, including Alzheimer’s Disease (AD). It was originally assumed that this may be a side effect of AD however, circadian disruptions can be detected very early in the disease and recently it has been suggested that they may actively contribute to AD. Although many studies have correlated circadian disruptions with memory decline and AD, how they are functionally linked remains elusive. We are therefore investigating how proteins involved in AD may cause circadian disruptions using the Drosophila model. We are focusing on the Amyloid Precursor Protein (APP) and the fragments that are produced from it, which include the Amyloid ß fragment that accumulates in the plaques that are characteristic for AD. We are testing whether APP is found in the master clock neurons that regulate circadian rhythms and that are responsible for synchronizing the rhythms in clocks found throughout the body and we are investigating how disease-associated changes in APP affect these neurons.