Xiao Lab
Xiao research
Our research is at the emerging interface of chemistry and biology by developing chemical tools to better treat and understand cancer. A particular focus in the lab is to develop chemical tools and potential cancer therapeutics targeting transcription process and DNA-repair pathways. Synopses of two ongoing projects are listed below:
1. Chemical inhibitors of CREB-mediated gene transcription
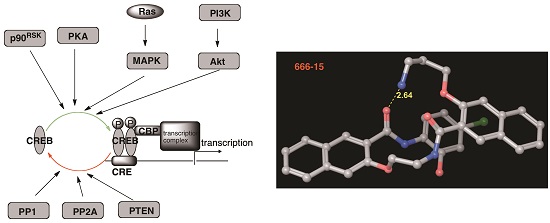
cAMP-response element binding protein (CREB) is a nuclear transcription factor. Its transcription activity is regulated by dynamic phosphorylation and dephosphorylation events. CREB is a signaling hub of many signaling transduction pathways that are often dysregulated in cancer cells. In cancer cells, CREB is often overexpressed and/or overactivated. Functional genetics studies demonstrated that intact CREB’s transcription activity is critical in maintaining cancer phenotype. We hypothesized that small molecule inhibitors of CREB would represent potential novel cancer therapeutics against multiple types of cancers (Curr Cancer Drug Targets, 2010). To test this hypothesis, we initiated a research program to identify small molecule inhibitors of CREB-mediated gene transcription. We have developed 666-15 as a very potent and cell-permeable CREB inhibitor (J Med Chem, 2015; J Med Chem, 2019). 666-15 not only inhibited cancer cell growth in vitro, but also showed robust in vivo anticancer activity. 666-15 has been an invaluable tool to investigate CREB biology and it is widely available from Tocris, Sigma, and Calbiochem.
2. Chemical tools to modulate nuclear lamin functions
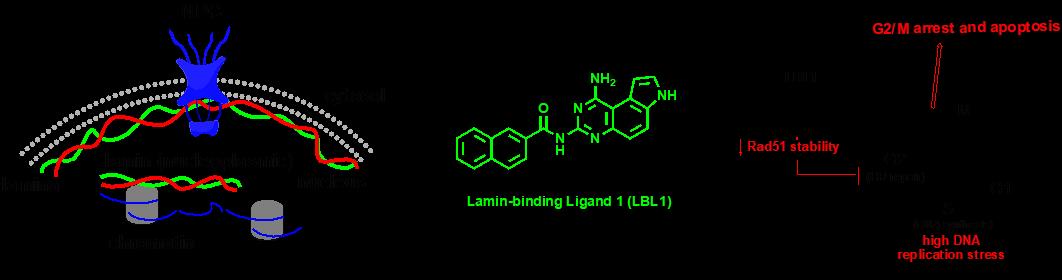
Nuclear lamins (LMNA, LMNB1, LMNB2 and LMNC) are type V intermediate filaments. They form nuclear lamina underneath the inner nuclear membrane. In addition to their structural roles to support the nuclear shape, they also present signaling functions through poorly understood mechanisms. For example, HGPS (Hutchinson-Gilford progeria syndrome) is caused by a point mutation in LMNA gene to activate a cryptic splicing site leading to premature aging. This mutant LMNA is hypothesized to disrupt the normal signaling function of wild type LMNA. Elucidation of lamins’ signaling mechanisms has been hampered by the lack of a chemical tool to directly modulate lamins. We are utilizing reverse and forward chemical genetics strategies to identify small molecule modulators of lamins (ACS Chem Biol, 2018). We recently discovered a novel function of LMNA in regulating the stability of the key DNA double-strand break repair protein Rad51. We have further developed LBL1 (Lamin-Binding Ligand 1) as the first small molecule to directly bind to nuclear lamins. Treatment of the cancer cells with LBL1 resulted in impaired homologous recombination (HR) repair and cell cycle arrest and eventual apoptosis (ACS Cent Sci, 2018). These compounds will provide critical tools to further our mechanistic understanding of lamins, which is poorly understood. They will also provide starting points for developing potential therapeutics for aging and cancer.
Recent publications
Find the full list of publications here: http://www.ncbi.nlm.nih.gov/pubmed/?term=xiangshu+xiao)
- Alkayed, N. J.;* Cao, Z.; Qian, Z.; Nagarajan, S.; Liu, X.; Nelson, J.; Xie, F.; Li, B. X.; Fan, W.; Liu, L.; Grafe, M. R.; Xiao, X.;* Barnes, A. P.;* Kaul, S.* “Bidirectional Control of Coronary Vascular Resistance by Eicosanoids via a Novel GPCR”, bioRxiv, DOI: 10.1101/420406. (*co-senior authors)
- Li, B. X.; Xiao, X.* “A suite of bioassays to evaluate CREB inhibitors” Methods in Enzymology 2020, doi.org/10.1016/bs.mie.2019.11.002.
- Xie, F.; Fan, Q.; Li, B. X.*; Xiao, X.* “Discovery of a Synergistic Inhibitor of cAMP-Response Element Binding Protein (CREB)-mediated Gene Transcription with 666-15” J. Med. Chem., 2019, 62, 11423-11429.
- Brown, P.; Xiao, X. (part of RELISH Consortium with >1000 authors); Zhou, Y.* “Large expert-curated database for benchmarking document similarity detection in biomedical literature search” Database, 2019, 1-66, doi.org/10.1093/database/baz085.
- Xiao, X.; Li, B. X.* “Identification of Lamins as the Molecular Targets of LBL1 using a clickable photoaffinity probe” Methods in Enzymology, 2019, doi.org/10.1016/bs.mie.2019.02.038.
- Jiang, M.; Yan, Y.; Yang, Kai, Liu, Z.; Qi, J.; Zhou, H.; Qian, N.; Zhou, Q.; Wang, F.; Wang, T.; Xu, X.; Xiao, X.; Deng, L. “Small molecule nAS-E targeting cAMP response element binding protein (CREB) and CREB-binding protein interaction inhibits breast cancer bone metastasis”, J. Cell. Mol. Med., 2019, 23, 1224-1234.
- Duque-Afonso, J. Lin, C. H. Han, K. Morgens, D. W. Jeng, E. E. Weng, Z. Jeong, J. Wong, S. H. K. Zhu, L. Wei, M. C. Chae, H. D. Schrappe, M. Cario, G. Duyster, J. Xiao, X. Sakamoto, K. M. Bassik, M. C. Cleary, M. L. “CBP modulates sensitivity to dasatinib in pre-BCR+ acute lymphoblastic leukemia” Cancer Res., 2018, 78, 6497-6508.
- Li, B. X.;* Chen, J.; Chao, B.; Zheng, Y.; Xiao, X.* “A Lamin-Binding Ligand Inhibits Homologous Recombination Repair of DNA Double-Strand Breaks” ACS Cent. Sci. 2018, 4, 1201-1210.
- Li, B. X.;* Chen, J.; Chao, B.; David, L; Xiao, X.* “A lamin-binding ligand LBL1 targets nuclear lamins, ACS Chem. Biol., 2018, 13, 1380-1387.


Xiangshu Xiao, Ph.D. (CV)
Xiangshu received his B.S. from then Beijing Medical University (now Peking University Health Science Center). He then joined a master graduate program at Shanghai Institute of Materia Medica in Chinese Academy of Sciences. In 2001, he joined Purdue University as Ph.D. student working with Prof. Mark Cushman. He was a postdoctoral fellow in the laboratory of Prof. Tom Kodadek at UT Southwestern Medical Center from 2005-2007 and joined OHSU faculty as an Assistant Professor in 2007.
Pedro Martín-Acosta, Ph.D.
Pedro graduated with Honours in Chemistry in 2013 from the University of La Laguna (ULL, Tenerife, Spain). A year later, he obtained his M.Sc. in Chemistry and joined the research group of Prof. Ana Estévez-Braun (2014) at the Antonio Gonzalez Institute for Bioorganic Research (IUBO-AG) at same University. He was awarded a research Fellowship FPI-ACIISI from government of the Canary Islands and got his Ph.D. in Organic and Medicinal Chemistry in 2019. His research was focused on synthesis and computational studies of new bioactive molecules against targets of pharmacological interest from natural and synthetic compounds through domino and/or multicomponent reactions. During his Ph.D. training he carried out several research stays at the research groups of Prof. Eelco Ruijter and R. Orru at the VU University Amsterdam (2016) and Prof. Phil S. Baran at The Scripts Research Institute (2017). In 2019 he joined the Xiao lab at OHSU where he has been working in the design and synthesis of PROTACs for CBP protein degradation.

Francis Dhoro, Ph.D.
Francis obtained his BS (with honors) in Chemistry from the University of Zimbabwe in 1999. His passion for further studies saw him in a chemistry graduate program at the University of Hawaii in 2002. At the University of Hawaii, Francis worked under the guidance of Professor Marcus A. Tius. His research was on the development of new methods for organic synthesis by means of a modified Nazarov cyclization. In 2006 Francis was awarded a Master of Science in Chemistry from the University of Hawaii. He then returned to Zimbabwe where he pursued a teaching career at a local university before moving to Perth to pursue his PhD in organic chemistry in 2015. Francis was awarded the International Postgraduate Research Scholarship (IPRS) and the Australian Postgraduate Award (APA) at the University of Western Australia. Whilst down under, Francis worked under the guidance of Professor Matthew Piggott. His research was directed at the total synthesis of highly condensed heteroaromatic natural alkaloids. Francis obtained his PhD in 2019. Inspired by the utility of synthetic chemistry in cancer research, Francis joined the Xiao lab in October 2019 as a postdoctoral fellow. Francis was awarded the NIH supplement to further his studies in cancer research. He is interested in medicinal chemistry related research. In his spare time Francis likes to read all kinds of books and watch sports.

Mark Miller, Ph.D.
Trained as a chemical biologist, Mark obtained his Ph.D. in Pharmaceutical and Chemical Sciences with an emphasis in Toxicology from the University of the Pacific in 2018. His graduate research focused on the development of AG10, a transthyretin stabilizer for use in the treatment of Familial Amyloid Cardiomyopathy, currently in clinical trials, as well as a technology to extend the half-life of peptides by leveraging transthyretin. After graduation, he joined Eidos Therapeutics as a scientist in order to assist in clinical assay development for the clinical trials of AG10. Then, he moved to Portland, Oregon to begin work at OHSU in the Xiao Lab as a Postdoctoral Researcher. Currently, he is working on analogues of previously identified CREB inhibitors.

Julia Warren, Ph.D.
Julia received her undergraduate degree in Chemistry at Whitman College in Walla Walla, WA. After spending a couple of years working, traveling and dabbling in marine conservation research, she enrolled in a master program at the University of Copenhagen. Upon completion of her M.Sc. in organic chemistry in 2016, she continued her work in artificial enzyme development and small molecule probes as a Ph.D. candidate under the supervision of Professor Mikael Bols at the University of Copenhagen. In 2019, Julia completed her Ph.D. and returned to her native Pacific Northwest to further pursue her interest in small molecule probe development as a member of the Xiao Lab at OHSU.