Partial agonists are receptor ligands that, under saturating conditions, nevertheless result in less than maximal activation. Despite extensive studies, the molecular insights into why partial agonists give rise to limited receptor activation remain elusive. The pentameric glycine receptor (GlyR), a hallmark Cys-loop receptor, mediates signal transduction at chemical synapses and transitions between resting, open, and desensitized states in responses to ligand–neurotransmitter–binding.
Gouaux lab members and first co-authors, Jie Yu and Hongtao Zhu, and their collaborators at the Van Andel Institute and University College London, carried out single channel electrophysiology and single particle cryo-EM studies to show how partial agonists populate agonist-bound yet closed channel states, providing the first structural insights into reduced efficacy of the agonists.
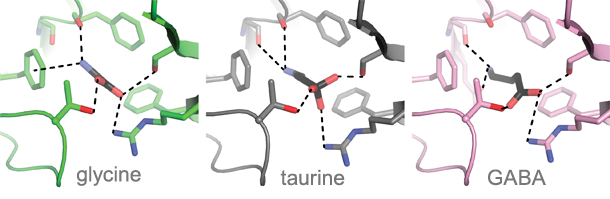
Their research also revealed structures of the receptor bound with agonist yet in a closed ion channel state and found that partial agonists produce less substantial conformational changes in the neurotransmitter binding pocket in comparison to full agonists, thus providing a metric to correlate the extent of agonist-induced conformational changes to open channel probability across the Cys-loop receptor family. With structures of agonist-bound closed, open and desensitized states in hand, the lab will proceed to map the conformational changes of the receptor as it transitions throughout the entire gating cycle.
The findings were published online February 9 in the journal Cell.