Featured Publication
In a new paper published in Science Advances, Dr. Steven Mansoor and Adam Oken, B.A., a graduate student in the Mansoor lab, used advanced imaging techniques to look at the structure of the P2X7 receptor once it’s bound to five known antagonists and a new one they discovered. Antagonists are molecules that bind to the receptor and prevent it from activating, effectively blocking receptor function. Antagonists against P2X7 receptor could be aimed at treating conditions resulting from neuroinflammation, vascular inflammation and even cancer. Read more in the OHSU News story and their paper in Science Advances.
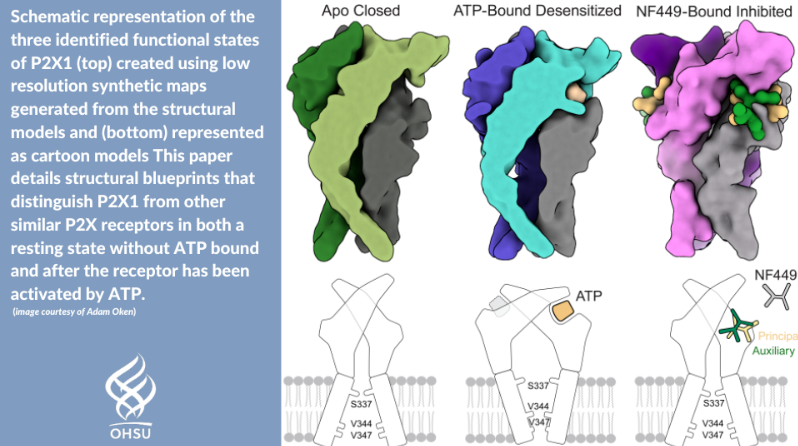
In the same week, the Mansoor lab published another paper in Nature Communications that document their findings on the P2X1 receptor, which they hope will lead to new therapies aimed at preventing blood clots. Read more about their work in the OHSU News story and their findings in Nature Communications.
The Mansoor lab would like to thank Barbara Allen, Jim Batzer, and Randy and Barbara Lovre for their generous support.
Prestigious award seeks to advance new avenue of addiction treatment

James Frank, Ph.D., assistant professor of chemical physiology and biochemistry in the OHSU School of Medicine, was awarded the prestigious Avenir Award from the National Institute on Drug Abuse. Dr. Frank will use the award to develop new tools to improve understanding of an alternative suite of receptors in the brain believed to be involved in opioid addiction. Dr. Frank’s project was one of just two Avenir Awards in Chemistry and Pharmacology Research granted this year. NIDA has three Avenir award programs: research in HIV, genetics and epigenetics of substance use, and chemistry and pharmacology of substance use. Only two OHSU scientists have received an Avenir in any category. Read more in the OHSU News story.
OHSU co-founded company, Autobahn Therapeutics, attracts $100 million investment

Autobahn Therapeutics — a biotechnology company first established out of a lab at Oregon Health & Science University — has generated a $100 million round of private investment to develop new treatments for people affected by neuropsychiatric disorders, such as depression, and eventually for other central nervous system disorders. Tom Scanlan, professor of chemical physiology and biochemistry in the OHSU School of Medicine, worked for decades toward the initial discovery of the compound, and to develop the technology that now underpins the company’s ABX-002 program. OHSU licensed the compound to Autobahn in 2018. Find out more in the OHSU News story.
OHSU researchers discover link between proteins in tears, pain after eye surgery

Sue Aicher, Ph.D., in the OHSU School of Medicine and Brooke Harkness, O.D., M.S., FAAO, assistant professor of ophthalmology at OHSU Casey Eye Institute, published a study in the Journal of Proteome Research, that shows a connection between levels of certain proteins found in patients’ tears and persistent pain months after refractive surgery. Find out more details at the OHSU News story.
Upcoming Events
Recent News
- Poster Prize Winner for the 30th International Symposium on Hepatitis C Virus, Flaviviruses, and Related Viruses Awarded to Judah Evangelista, Ph.D.
- OHSU School of Medicine GSO Diversity, Equity, and Inclusion Leadership Awarded to Assmaa ElSheikh
- American Physiological Society Research Recognition Awarded to Arianna Scalco, Ph.D.
- CPB Welcomes New Faculty!
- American Heart Association (AHA) Postdoctoral Fellowship Awarded to Satyaki Chatterjee, Ph.D.
CPB Diversity Statement

The Department of Chemical Physiology & Biochemistry is committed to a workplace that promotes and values diversity. We believe that prioritizing inclusion and equity is key to the wellbeing of our members and fundamental to an excellent research and training environment. We are taking active measures to eliminate biases and inequities that have historically excluded people from the biomedical research community. We embrace our differences and recognize that diversity enriches our community and strengthens our scientific endeavors. Click here to learn more.