Kelly Lab
Gonadal and metabolic hormone modulation of hypothalamic neuronal excitability and control of homeostasis
The female gonadal steroid 17β-estradiol is a pleiotropic hormone that has widespread actions not only in reproductive tissues but also has pronounced effects in the central nervous system (CNS). For over thirty years my laboratory has been studying the actions of estradiol in the CNS, specifically in hypothalamic neurons that control homeostasis and behavior using molecular, electrophysiological and more recently optogenetic techniques.
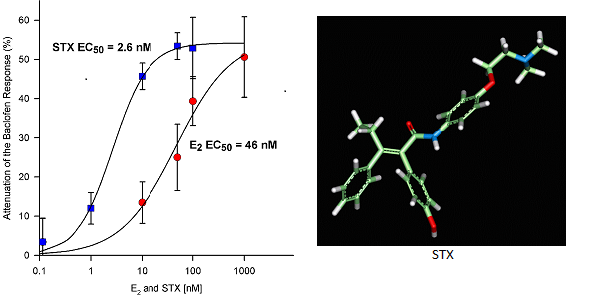
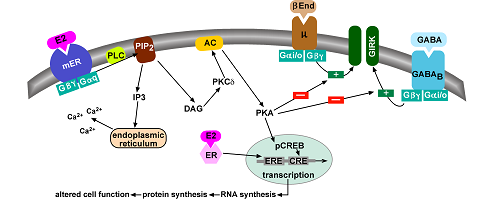
In collaboration with Drs. Oline Ronnekleiv and Tom Scanlan in the Department, we developed a novel non-steroidal compound, STX that mimics the rapid membrane actions of estradiol in hypothalamic neurons (Figure 1). We discovered that STX and estradiol act directly on these neurons via a G protein-coupled receptor to alter their activity. This unique membrane-associated estrogen receptor is Gq-coupled to activation of a phospholipase C-protein kinase C-protein kinase A pathway leading to desensitization of μ-opioid and GABAB receptors in POMC neurons (Figure 2), but sensitization of these Gαi,o-coupled receptors in NPY/AgRP neurons. We use two different experimental models. One model is the female guinea pig whose reproductive cycle mimics that of the human and therefore is ideal for doing whole-animal experiments (see below). Another model is the transgenic female mouse in which we can target POMC-Cre and NPY/AgRP-Cre (cells express green fluorescence protein) for whole cell recording and also deliver viral vectors to express channel-rhodopsin for elucidating downstream synaptic targets that are responsive to gonadal and metabolic hormones.
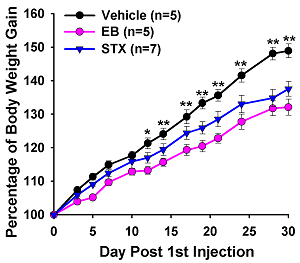
As proof of principle, estradiol is known to control feeding behavior, energy homeostasis and core body temperature. STX appears to mimic all of these CNS actions of estrogen in female rodents (Figures 3 & 4). In addition, estradiol has been known to have neuroprotective effects on higher cognitive functions. Indeed, in collaboration with colleagues at Albert Einstein College of Medicine, we have found that acute STX is as efficacious as estradiol to prevent hippocampal neuron loss following global ischemia in middle aged rats. Therefore, STX appears to mimic many of the "neuroprotective" actions of estradiol in the CNS.
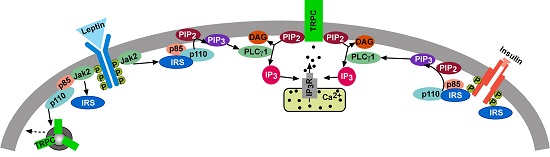
Since we have recently discovered that insulin and leptin depolarize and excite POMC neurons via activation of TRPC channels (Figure 5), we are investigating how the Gαq-mER signaling pathway cross-talks with insulin and leptin signaling in POMC neurons to promote its actions and protect against the development of insulin resistance in obese states.
Key References:
- Kelly MJ, Qiu J. Estrogen signaling in hypothalamic circuits controlling reproduction. Brain Res. 1364:44-52, 2010. PMC3070154
- Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlova-Wuttke D, Wuttke W, Scanlan TS, Rønnekleiv OK, Kelly MJ. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology 151: 4926-37, 2010. PMC2946146
- Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. The Journal of Neuroscience 30:1560-5, 2010 : PMC20107083.
- Lebesgue D, Traub M, De Butte-Smith M, Chen C, Zukin RS, Kelly MJ, Etgen AM. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats. PLoS One. 2010 Jan 8; 5 (1):e8642. PMC2799530
- Roepke TA, Qiu J, Smith AW, Rønnekleiv OK, Kelly MJ. Fasting and 17β-estradiol differentially modulate the M-current in Neuropeptide Y neurons. The Journal of Neuroscience 31:18825-35, 2011. PMC3243733
- Qiu J, Fang Y, Bosch MA, Rønnekleiv OK, Kelly MJ. Guinea Pig Kisspeptin Neurons Are Depolarized by Leptin via Activation of TRPC Channels. Endocrinology. 2011 Apr; 152 (4) :1503-14, 2011. PMC3078701
- Roepke TA, Qiu J, Smith AW, Rønnekleiv OK, Kelly MJ. Serotonin 5-HT2c receptor-mediated inhibition of the M-current in hypothalamic POMC neurons. Am J Physiol Endocrinol Metab 302:E1399-E1406, 2012. PMC 3378066
- Kelly MJ, Rønnekleiv OK. Membrane-initiated actions of estradiol that regulate reproduction, energy balance and body temperature. Frontiers in Neuroendocrinology 33:: 376-387, 2012. PMC3618441.
- Smith A, Bosch MA, Wagner EJ, Rønnekleiv OK, Kelly MJ. The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: Role in mediating the anorexigenic effects of 17β-estradiol. Am. J. Physiol. Endocrinol. Metab. 305: E632-40, 2013. PMC 3761166
- Qiu J, Qiu J, Zhang C, Borgquist A, Nestor CC, Smith AW, Bosch MA, Ku S, Wagner EJ, Rønnekleiv OK, Kelly MJ. Insulin excites anorezigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metabolism 19:682-693, 2014. PMC4183666