Blackburn Lab
About Us
Our lab focuses on the structure and function of oxidase and oxygenase metalloenzymes, particularly copper monooxygenases. We specialize in coordination chemistry and the biochemistry of copper, with particular focus in enzymes which influence neuroendocrine function, pathogen virulence, and cancer. Our biophysical areas of expertise include X-ray absorption spectroscopies (XAS, EXAFS, X-ray crystallography), spectroscopy (EPR, UV-Vis, fluorescence, FTIR), and rapid kinetics (RFQ, stopped-flow, quench-flow microvolume).
People

Professor Ninian Blackburn
Ph.D., 1975, University of Dundee, Scotland
Specialties:
- Biochemistry of metalloproteins, particularly on the role of copper in neuroendocrine function and investigation of metal ion homeostasis
- Advanced X-ray spectroscopies (XAS, EXAFS, XES)
- Coordination chemistry
- Electron paramagnetic resonance (EPR)
- Fourier-transform infrared (FTIR) spectroscopy

Dr. Renee Arias
Ph.D., 2018, California Institute of Technology, Pasadena, CA
B.S., 2012, University of Oregon, Eugene, OR
Specialties:
- Investigation of the structure and function of metalloproteins, with emphasis on FeS and Cu proteins
- X-ray crystallography
- Anaerobic protein purification (liquid chromatography, ion exchange, metal reconstitution)
- Rapid kinetics and analysis: stopped-flow (SF), microvolume quench-flow (QFM), HPLC
- X-ray spectroscopy (XAS, HERFD)

Dr. Katie Rush
Ph.D., 2018, University of Michigan, Ann Arbor, MI
B.S., 2013, University of Tennessee, Knoxville, TN
Specialties:
- Biochemical investigation of metalloproteins, with emphasis on Hg, FeS, and Cu systems
- X-ray spectroscopies (XAS, HERFD)
- Rapid kinetics: rapid freeze-quench (RFQ)
- Electron paramagnetic resonance (EPR)
- Protein purification (liquid chromatography, ion exchange)
Dr. Rush is co-advised by Professor Blackburn at OHSU, and Professor Kelly Chacón at Reed College

Katherine Alwan
M.S., 2015, Oregon Health and Science University, Portland, OR
B.A., 2014, Skidmore College, Saratoga Springs, NY
Specialties:
- Use of rationally designed protein scaffolds to investigate peptidylglycine alpha-hydroxylating monooxygenase active site function
- X-ray spectroscopies (XAS)
- Electron paramagnetic resonance (EPR)
- Fourier transform infrared (FTIR) spectroscopy
- Protein purification
- Kinetics evaluation (SigmaPlot)

Evan Welch
B.A., 2017, Reed College, Portland, OR
Specialties
- Investigation of peptidylglycine alpha-amidating monooxygenase function using biochemical and structural methods
- Protein purification (liquid chromatography, ion exchange, metal reconstitution)
- Fourier transform infrared (FTIR) spectroscopy
- Rapid kinetics and analysis: microvolume quench flow (QFM), HPLC
- Kinetics evaluation and programming: DynaFit, SigmaPlot, R, Python

Ben Gambill
B.S., 2014, Oregon State University, Corvallis, OR
Specialties:
- Mammalian cloning and cell culture
- General lab maintenance
Research Projects
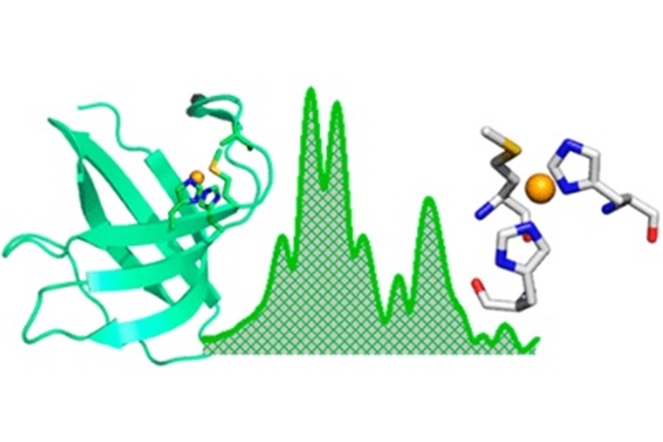
Metallochaperone scaffold proteins as models to investigate copper monooxygenase structure and function.
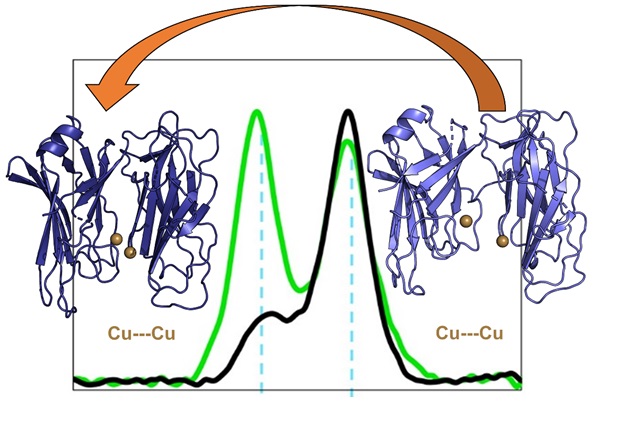
Investigation of peptidylglycine alpha-amidating monooxygenase chemical structure and reactivity.
Research Specialties
- Advanced X-ray spectroscopies: X-ray absorption (XAS) and Extended X-ray Absorption Fine Structure (EXAFS)
- X-ray crystallography
- Fourier transform infrared (FTIR) spectroscopy
- UV-visible spectroscopy (UV-vis)
- Electron paramagnetic resonance (EPR)
- Fast kinetics: stopped-flow (SF), rapid freeze-quench (RFQ), quench flow microvolume (QFM)
Recent Publications
pH-Induced Binding of the Axial Ligand in an Engineered CuA Site Favors the πu State.
Morgada MN, Emiliani F, Chacón KN, Álvarez-Paggi D, Murgida DH, Blackburn NJ, Abriata LA, Vila AJ. Inorg Chem. 2019 Dec 2;58(23):15687-15691. doi: 10.1021/acs.inorgchem.9b01868. Epub 2019 Nov 11. PubMed PMID: 31710470.
Alwan KB, Welch EF, Blackburn NJ. Biochemistry. 2019 Nov 5;58(44):4436-4446. doi: 10.1021/acs.biochem.9b00823. Epub 2019 Oct 28. PMID: 31626532
Rao G, Pattenaude SA, Alwan K, Blackburn NJ, Britt RD, Rauchfuss TB. Proc Natl Acad Sci U S A. 2019 Oct 15;116(42):20850-20855. doi: 10.1073/pnas.1913324116. Epub 2019 Sep 30. PubMed PMID: 31570604; PubMed Central PMCID: PMC6800375.
Rao G, Alwan KB, Blackburn NJ, Britt RD. Inorg Chem. 2019 Oct 7;58(19):12601-12608. doi: 10.1021/acs.inorgchem.9b01293. Epub 2019 Sep 20. PubMed PMID: 31539235; PubMed Central PMCID: PMC6984664.
Alwan KB, Welch EF, Arias RJ, Gambill BF, Blackburn NJ. Biochemistry. 2019 Jul 16;58(28):3097-3108. doi: 10.1021/acs.biochem.9b00312. Epub 2019 Jun 27. PMID: 31243953
Trapping intermediates in metal transfer reactions of the CusCBAF export pump of Escherichia coli.
Chacón KN, Perkins J, Mathe Z, Alwan K, Ho EN, Ucisik MN, Merz KM, Blackburn NJ. Commun Biol. 2018 Nov 14;1:192. doi: 10.1038/s42003-018-0181-9. eCollection 2018. PMID: 30456313
Kline CD, Blackburn NJ. Biochemistry. 2016 Dec 6;55(48):6652-6661. Epub 2016 Nov 22. PMID: 27933800
Kline CD, Gambill BF, Mayfield M, Lutsenko S, Blackburn NJ. Metallomics. 2016 Aug 1;8(8):729-33. doi: 10.1039/c6mt00062b. PMID: 27242196
Chauhan S, Hosseinzadeh P, Lu Y, Blackburn NJ. Biochemistry. 2016 Apr 5;55(13):2008-21. doi: 10.1021/acs.biochem.6b00061. Epub 2016 Mar 23. PMID: 26982589
Martin-Diaconescu V, Chacón KN, Delgado-Jaime MU, Sokaras D, Weng TC, DeBeer S, Blackburn NJ. Inorg Chem. 2016 Apr 4;55(7):3431-9. doi: 10.1021/acs.inorgchem.5b02842. Epub 2016 Mar 11. PMID: 26965786
Fun Science!
Gordon Research Conference 2020


Crystal images
Team copper represent overseas
