Cyclic Immunostaining for Multicolor Microscopy
Multicolor Microscopy to Understand Cancer & Improve Therapy
Multiplexed imaging technologies to simultaneously visualize numerous proteomic molecules in situ would significantly improve understanding of cancer heterogeneity, overlapping signaling pathways and dynamic interactions with the microenvironment. The maximum number of fluorophores that can be visualized in a single sample using conventional fluorescence microscopy is five and using unmixing techniques is improved to seven. To improve spectral resolution of immunostaining, various cyclic immunofluorescence (IF) and immunohistochemical (IHC) methods using cycles of fluorescent tagging, imaging and bleaching or dissociation of the affinity tags have been developed increasing multiplexing capabilities by serially imaging a sample that is re-stained many times. Tissue imaging using mass spectroscopy has also demonstrated significant multiplexing capabilities. However, current cylic IF and IHC (cycIF/IH) and mass spectroscopy imaging techniques have distinct disadvantages including harsh treatments to remove fluorescence signal or antibody, which affects the integrity of the tissue as cycle number increases hindering co-registration, steric hindrance that can impede antigen detection as antibodies are added in subsequent cycles, and difficulty with integration into the routine workflow of clinical histopathology.
Oligonucleotide Conjugated Antibodies for CycIF/IHC
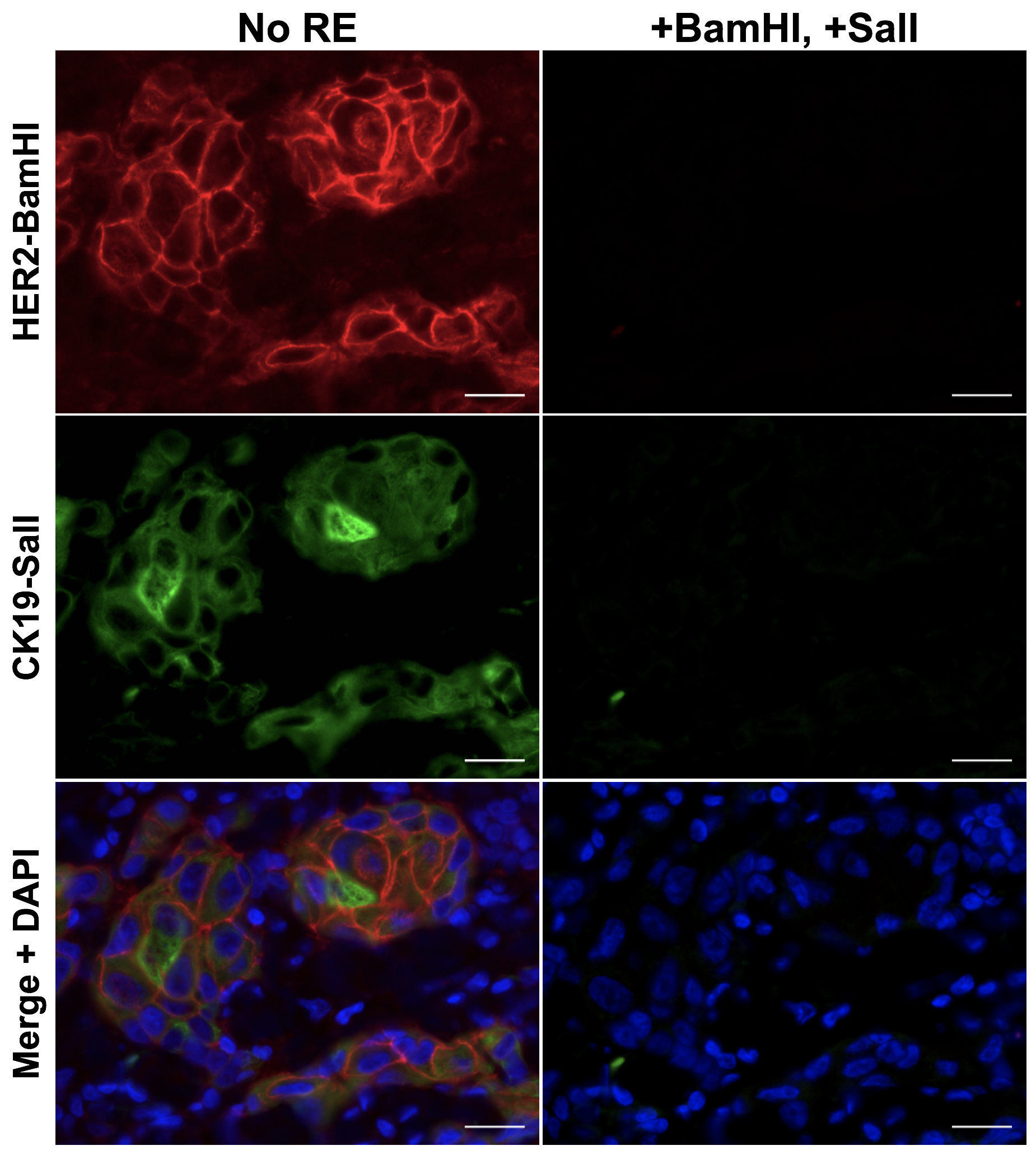
To facilitate compatibility with clinical histopathology, we have developed a novel cycIF/IHC technology based on antibody conjugated oligonucleotides (Ab-oligos). In our technology, a single stranded oligo (docking strand, DS) is conjugated to the primary antibody. A complementary single stranded oligo (imaging strand, IS) is conjugated to a conventional fluorophore, facilitating specific on tissue fluorescence labeling through hybridization, which can be imaged using any conventional fluorescence microscope. Each DS/IS pair was designed to contain a restriction enzyme (RE) site for selective removal of fluorescence permitting cycles of re-staining and imaging. The advantages of our method over other multiplexed methods include (1) all Ab-oligos can be applied in a single, mixed cocktail, preventing steric hindrance, (2) no effect on tissue integrity or antigenicity is seen following RE treatment, preserving tissue staining patterns over multiple cycles, and (3) mild tissue treatment conditions lend themselves to automation and integration into clinical histopathology workflow. This technology is being collaboratively developed with Dr. Joe Gray's laboratory to facilitate cycIF/IHC that will be compatible with conventional histology permitting integration into the clinical workflow.
Cyclic Immunostaining Figure