Special focus
Encompassing in vivo model conduct for collaborative science
Our highly collaborative team can support all aspects of macaque models from securing IACUC/IBC approval, to animal selection, in vivo study conduct (substance/pathogen preparation/administration, sampling, etc.), necropsy, ex vivo and in vitro processing, shipping, etc. This includes complex models requiring research related preventive and therapeutic veterinary interventions to optimize outcomes, and/or large studies involving dozens of macaques and all the associated logistics of ensuring consistent timing and utilizing high throughput procedures.
Comprehensive Sampling
We have the ability to sample an array of tissues in a minimally invasive fashion, do so repeatedly, with high throughput, and can perform these in combination without the need for antibiotics or anti-inflammatories that could impact experimental outcomes. Tissues include laparoscopic surgeries (liver partial lobectomies, hepatic lymph nodes, spleen biopsies, liver pinch biopsies, mesenteric and other abdominal lymph nodes), submandibular lymph nodes, axillary/inguinal lymph nodes, mucosal samples (GI, oral, vaginal), bone marrow, CSF, BAL, blood and more in development.
Macaque model development/optimization
Our veterinarians are specialists in developing new and optimizing existing macaque models to optimally answer key experimental questions and we can work with a host of different pathogens, perform reagent prep, flow cytometry, and ship samples as well to facilitate collaborations with offsite investigators.
Imaging
We can perform Near InfraRed (NIR) imaging via laparoscopy, and during open procedures and necropsies as well as extensive colonoscopy and image analysis.
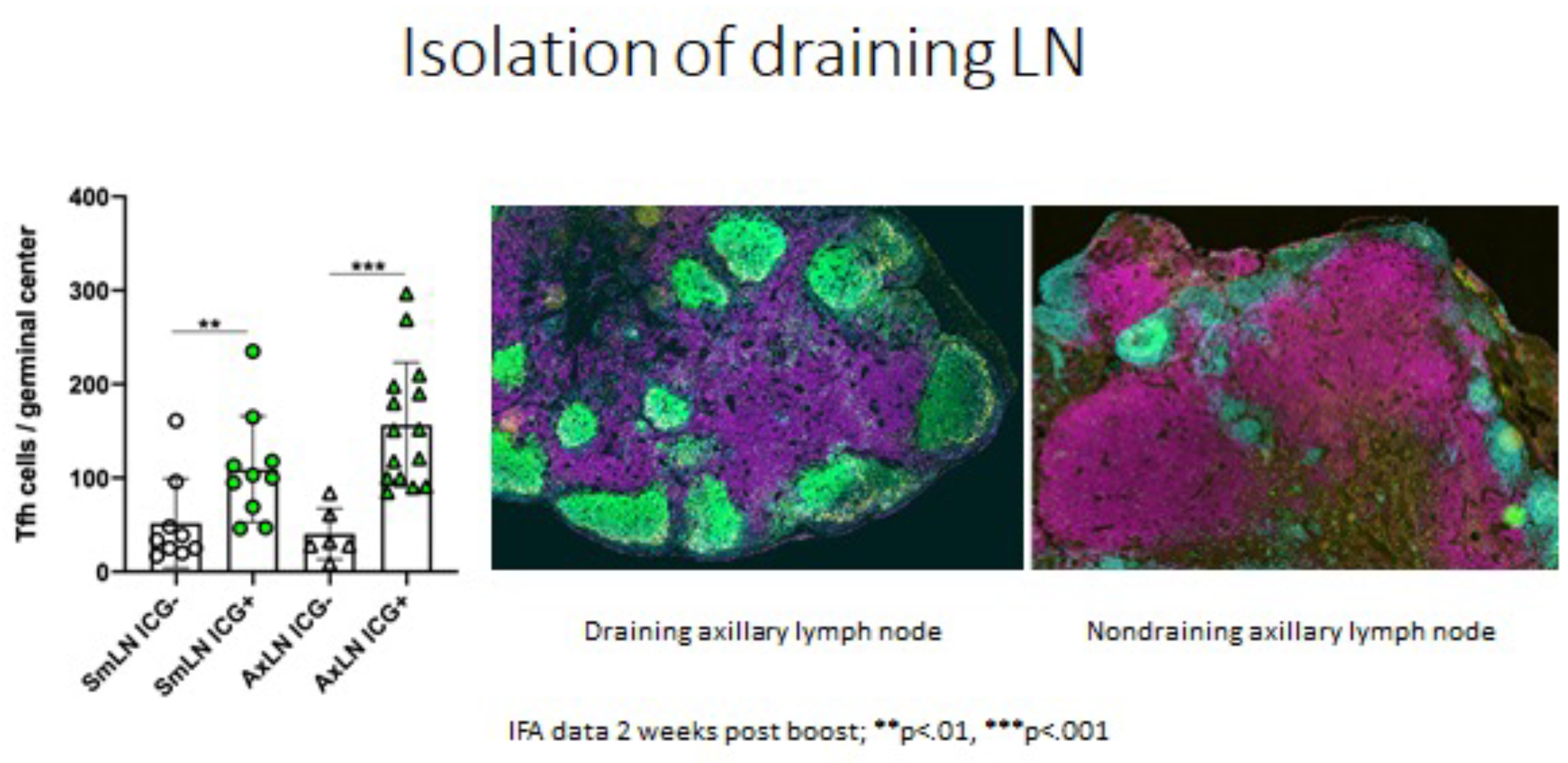
The importance of guided sampling can be seen below with identification of the axillary or submandibular LN draining the site of vaccination vs an adjacent LN collected from the same site on the same animal. The draining node exposed to the vaccine immunogen has more T follicular helper cells indicating that it is responding to the vaccine.
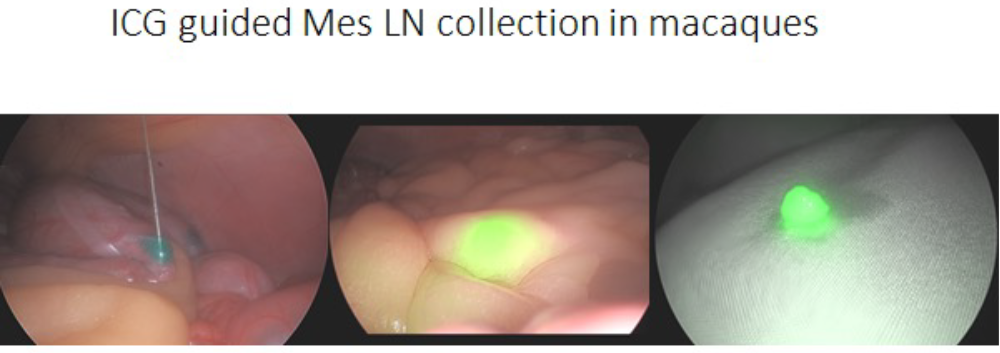
We have the same ability to perform guided LN sampling for abdominal LNs as well. In the example below mesenteric LN draining a specific location in the colon are identified.
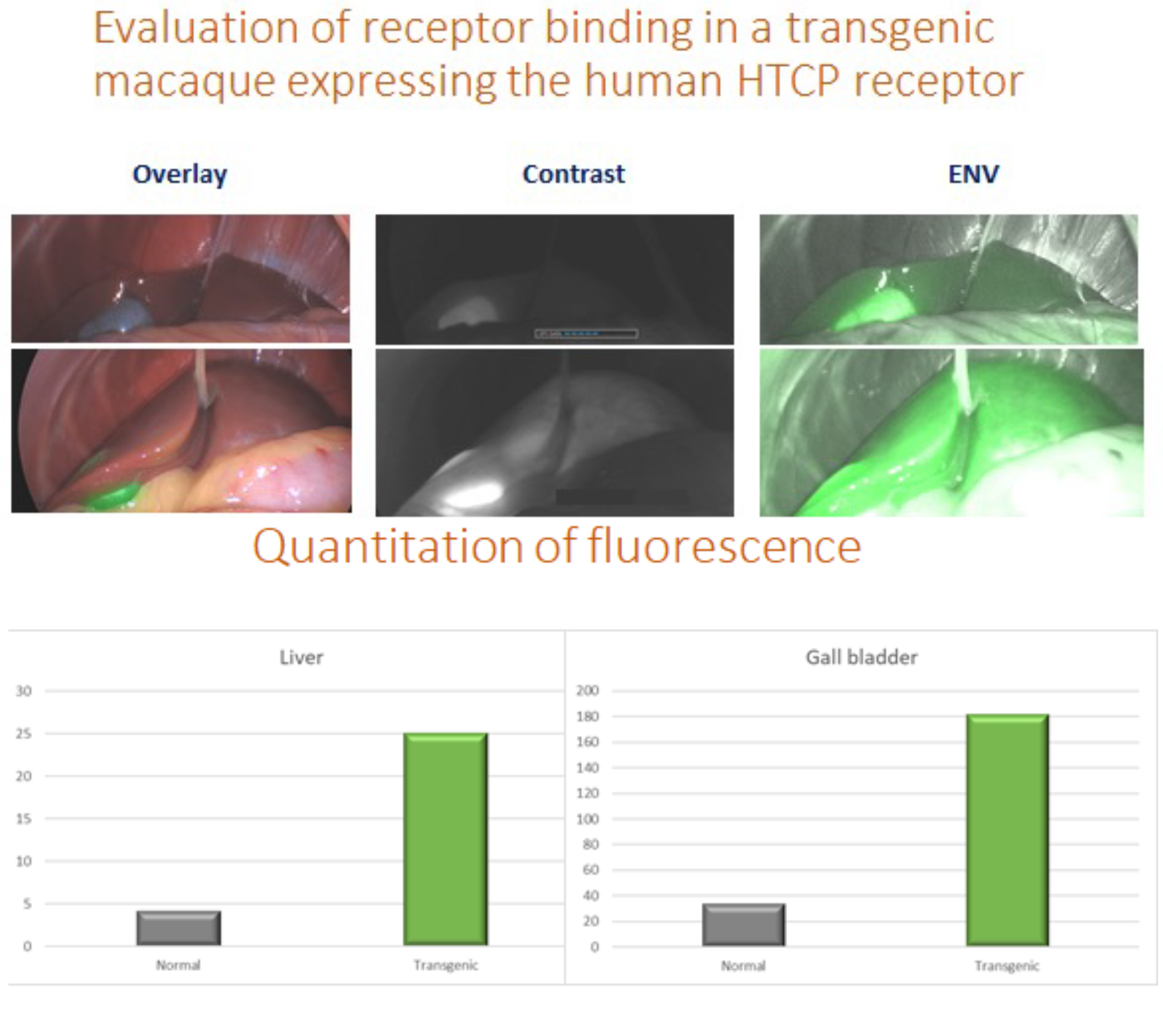
We can also evaluate and quantitate the amount of fluorescence from our NIR imaging platform. Below is an example from our Hepititis B model looking at binding of ICG labeled MYR-B that binds to the human NTCP receptor used by Hep B to enter cells. The top animal is a typical rhesus that lacks the HCTP receptor and the bottom animal is a transgenic with the fully functional HCTP receptor making it susceptible to Hep B infection. The distribution and density of the receptor can be quantitated by image analysis.