Research
Structural basis of KATP channel assembly, gating and pharmacology
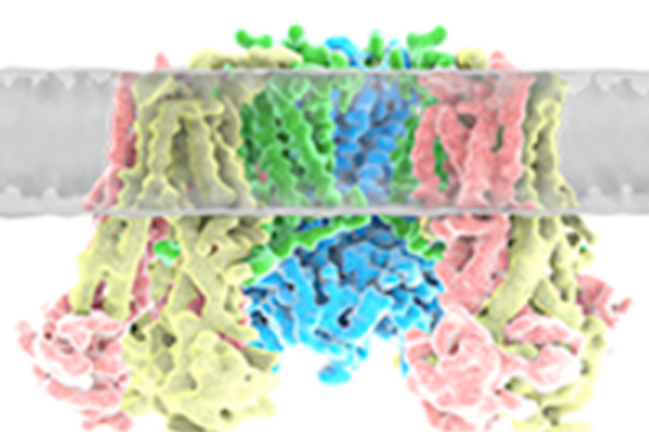
KATP channel is an obligatory hetero-octamer of four pore-forming Kir6 subunits and four regulatory sulfonylurea receptor (SUR) subunits. Both subunits are required for channel activity and response to physiological and pharmacological ligands. We are interested in understanding how the two protein subunits interact with each other and with ligands to function as a metabolic sensor, using a combination of single-molecule cryoEM, crosslinking mass spectrometry, molecular dynamics simulation, electrophysiology and biochemical techniques.
Selected publications:
- Sung, M.W., Z.Y. Yang, C.M. Driggers, B.L. Patton, B. Mostofian, J.D. Russo, D.M. Zuckerman, and S.-L. Shyng. Vascular KATP channel structural dynamics reveal regulatory mechanism by Mgnucleotides. (2021). PNAS, Nov 2;118(44):e2109441118. doi: 10.1073/pnas.2109441118.
- Martin, G.M., M.W. Sung, Z. Yang, L. M. Innes, B. Kandasamy, L.L. David, C. Yoshioka and S.-L. Shyng. (2019). Mechanism of pharmacochaperoning in a mammalian KATP channel revealed by cryo-EM. Elife, Jul. 25; 8. pii: e46417. doi: 10.7554/eLife.46417. PMC6699824.
- Martin, G.M., B. Kandasamy, C. Yoshioka, and S.-L. Shyng. (2017). Anti-diabetic drug binding site in a mammalian KATP channel revealed by Cryo-EM. Elife, Oct. 16; 6. pii: e31054. doi: 10.7554/eLife.31054. PMC5655142.
- Martin, G.M., C. Yoshioka, E.A. Rex, J. F. Fay, Q. Xie, M.R. Whorton, J.Z. Chen, and S.-L. Shyng. (2017). Cryo-EM structure of the ATP-sensitive potassium channel illuminates mechanisms of assembly and gating. Elife, Jan. 16; 6. pii: e24149. doi: 10.7554/eLife.24149. PMC5344670. First posted in bioRxiv on Dec. 16, 2016.
*Webpage image: a close look at a closed channel, March 9, 2017; accompanying “Insight” editorial piece “From ions to insulin” by Voula Kanelis, eLife, 6: e25159. Recommended for F1000Prime.
Signaling mechanisms governing insulin secretion in pancreatic β-cells
This project investigates how the adipocyte-derived hormone leptin regulates the trafficking of KATP channels in pancreatic β-cells to control insulin secretion and glucose homeostasis. Using biochemical, electrophysiology, biosensor-based live cell imaging, phosphoproteomic and BioID approaches, we are exploring the signaling pathway underlying the effect of leptin, in particular the role of NMDA receptors in mediating the effect of leptin to activate the downstream molecule AMPK, and how AMPK activation leads to PKA activation and actin reorganization to promote channel trafficking to the cell surface.
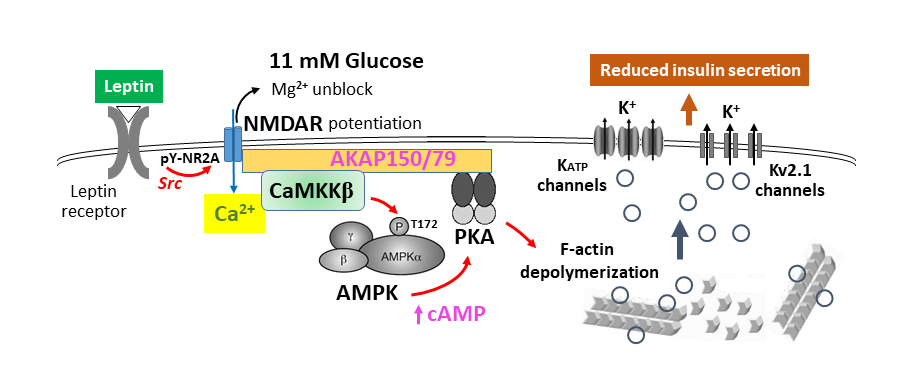
Selected publications:
- Cochrane VA, Yang Z, Dell’Acqua ML, Shyng SL. AKAP79/150 coordinates leptin-induced PKA signaling to regulate KATP channel trafficking in pancreatic β-cells. (2021). J. Biol. Chem. 296:100442.
- Cochrane VA, Wu Y, Yang Z, ElSheikh A, Dunford J, Kievit P, Fortin DA, Shyng SL. Leptin modulates pancreatic β-cell membrane potential through Src kinase-mediated phosphorylation of NMDA receptors. (2020). J. Biol. Chem. 295:17281-17297.
- Wu, Y., D.A. Fortin, V.A. Cochrane, P.-C. Chen, and S.-L. Shyng. (2017). NMDA receptors mediate leptin signaling and regulate potassium channel trafficking in pancreatic β-cells. J. Biol. Chem. 292: 15512-24. PMCID:PMC5602408.
- Wu, Y., S.-L. Shyng*, and P.-C. Chen*. (2015). Concerted trafficking regulation of Kv2.1 and KATP channels by leptin in pancreatic β-cells. J. Biol. Chem. 290: 29676-90. *Co-corresponding authors, PMCID:PMC3837151. JBC Paper of the Week, featured in JBC paper of the week podcast.
- Chen, P.-C., Y.N. Kryukova and S.-L. Shyng. (2013). Leptin regulates KATP channel trafficking in pancreatic β-cells by a signaling mechanism involving AMPK and PKA. J. Biol. Chem. 288(47):34098-109. PMCID:PMC3837151. *Journal cover, JBC Paper of the Week.
KATP channels and human disease
Mutations in KATP channels are linked to a number of human diseases. Loss of function mutations in ABCC8 encoding SUR1 or KCNJ11 encoding Kir6.2 underlie congenital hyperinsulinism whereas gain of function mutations cause neonatal diabetes and DEND syndrome. We are interested in understanding how mutations in KATP channel genes disrupt channel biogenesis and gating to cause disease. We have collaborated with clinical endocrinologists around the world to build a knowledge base of genotype-phenotype correlations in patients with KATP channel mutations. Our studies to date have uncovered many detailed mechanisms by which mutations alter channel gating and expression to result in the disease phenotypes observed in patients. This information has significant real-life impact on disease diagnosis and treatment (please check out the Congenital Hyperinsulinism International website).
Selected publications:
- Jacobson DA, Shyng SL. (2020). Ion channels of the islets in type 2 diabetes. J. Mol. Biol. 432: 1326-1346. Doi: 10.1016/j.jmb.2019.08.014.
- Balamurugan, K., B. Kavitha, Z. Yang, Y. V. Mohan, V. Radha, and S.-L. Shyng. (2019). Functional characterization of activating mutations in the SUR1 (ABCC8) causing neonatal diabetes mellitus in Asian Indian Children. Pediatr. Diabetes, 20(4):397-407. PMID:30861254.
- Martin GM, Rex E, Devaraneni P, Denton JS, Boodhansingh KE, DeLeon DD, Stanley CA, Shyng SL. (2016). Pharmacological correction of trafficking defects in ATP-sensitive potassium channels caused by sulfonylurea receptor 1 mutations. J. Biol. Chem. 291:21971-83. *Selected to appear in a special virtual issue on Ion Channels at JBC.
- Pinney S, Lin YW, MacMullen C, O’Rourke S, Hanna C, Shyng SL, Stanley CA. (2008). Clinical and biochemical features of congenital hyperinsulinism associated with dominant KATP channel mutations. J. Clin. Invest., 118: 2877-2886.
- Cartier, E. A., L.R. Conti, C.A. Vandenberg and S.-L. Shyng. (2001). Defective trafficking and function of KATP channels caused by a sulfonylurea receptor 1 mutation associated with persistent hyperinsulinemic hypoglycemia of infancy. (2001). Proc. Natl. Acad. Sci. USA, 98: 2882-7. PMC30234
KATP channel drug discovery
KATP channels have long been drug targets for a number of human diseases. For example, KATP channel inhibitors sulfonylureas are widely used to treat type 2 diabetes and neonatal diabetes. KATP channel activators such as diazoxide and monoxidil are used to treat congenital hyperinsulinism and hair loss (monoxidil is the main ingredient in Rogaine). However, these drugs lack isoform specificity, causing serious side effects. Using channel structures we have solved, we are conducting virtual screening of large compound libraries followed by functional screen to identify new KATP channel modulators with improved potency, efficacy and specificity.
Selected publications:
- Martin GM, Sung MW, Shyng SL. (2020). Pharmacological chaperones of ATP-sensitive potassium channels: Mechanistic insight from cryoEM structures. Mol. Cell. Endocrinol. 502:110667. Doi: 10.1016/j.mce.2019.110667.
- Devaraneni P, Martin GM, Olson EM, Shyng SL. (2015). Structurally distinct ligands rescue biogenesis defects of the KATP channel complex via a converging mechanism. J. Biol. Chem., 290: 7980-91.
- Chen, P.-C., E.M. Olson, Q. Zhou, Y. Kryukova, H. M. Sampson, D.Y. Thomas and S.-L. Shyng. (2013). Carbamazepine as a novel small molecule corrector of trafficking-impaired ATP-sensitive potassium channels identified in congenital hyperinsulinism. J. Biol. Chem., 288: 20942-54. PMC3774364.