Projects
Please take some time to read about the major projects underway in the Worth Lab. We collaborate extensively with the Rosie Sears Lab, the Jonathan Brody Lab, and the Brenden-Colson Center for Pancreatic Care, as well as other labs in the Knight Cancer Institute here at OHSU.
Understanding a devastating problem
The Worth Lab's main focus is on understanding the mechanisms underlying the rapid metastatic recurrence of pancreatic cancer after surgery. Both chemotherapy and surgery are interdependently necessary for the treatment of pancreatic cancer. Unfortunately, of those patients who undergo a surgical resection of their tumor, 20-30% of patients experience a distant recurrence of their disease within six months of treatment.
Our lab has been awarded several grants for the study of rapid recurrence with our novel in-vivo model which allows us to better investigate the origins and causes of this poorly understood phenomenon. Our goal is to understand the ways in which surgical resection may drive metastasis or cause dormant lesions in the liver to begin growing. As the mechanisms of this devastating clinical finding are poorly understood, the main focus of our lab is in understanding rapid recurrence in order to drive further translational and clinical investigations aimed at improving outcomes in the treatment of pancreatic cancer.
Our understanding of rapid recurrence takes on possible etiologies via several hypotheses:
- Occult synchronous lesions: single or colonies of cells that exist as metastases in the liver at the time of surgery. Are these lesions kept 'in check' by the immune system? If so, how? And how does surgery affect their outgrowth?
- Seeded metachronous lesions: cells that are circulating at the time of, or disseminated by, surgical intervention. It is known that even in early-stage pancreatic lesions, circulating cancer cells can be identified in the bloodstream. Is there something about surgical intervention in rapid-recurrence patients that primes the liver for metastasis? If so, can we prevent this?
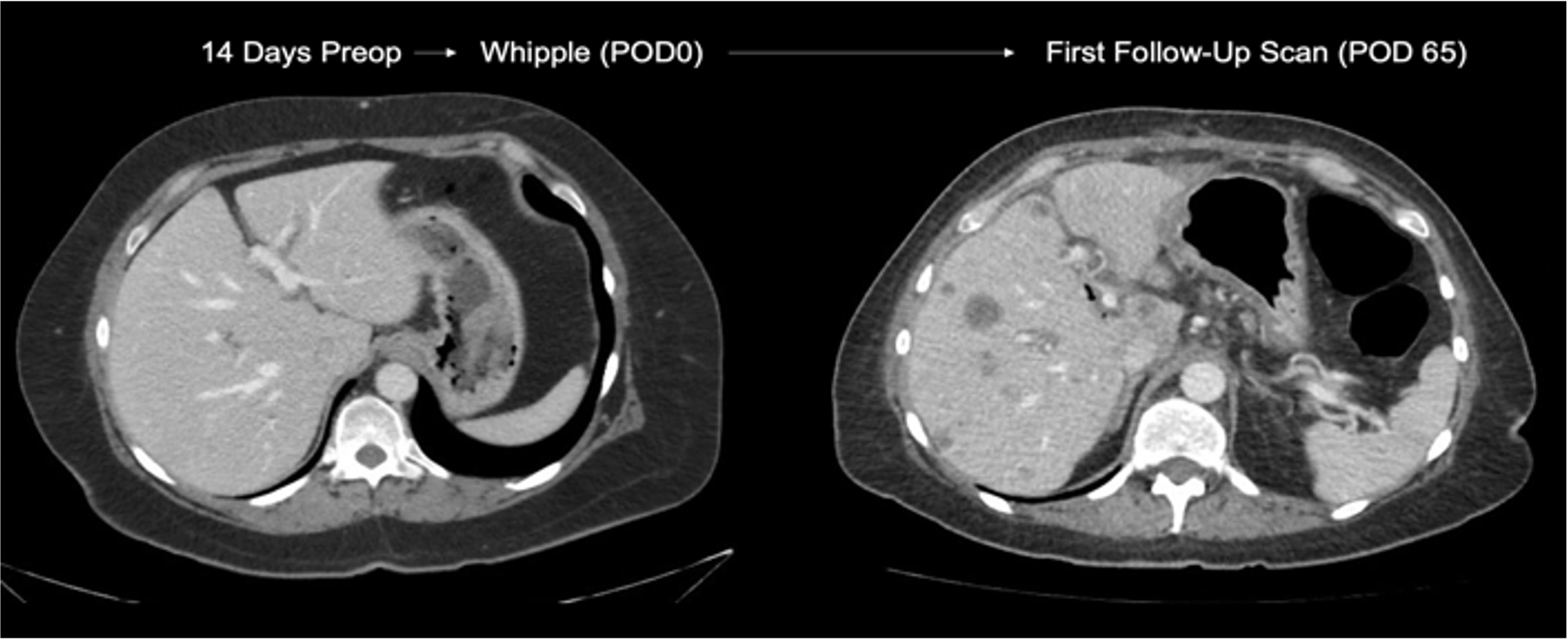
Through an OHSU Faculty Initiatives Grant 2021, the Worth Lab led an effort by multiple South Waterfront Campus labs at RLSB and KCRB to expand the imaging platforms available to South Waterfront researchers. Located in the RLSB Small Animal Imaging Core, the SkyScan 1278 is an ultra-low dose CT scanner capable of imaging down to 50um per voxel, which allows for astounding resolution at top speeds, thereby reducing the time needed to detect metastases in our preclinical models.
With this platform, we are able to perform extensive characterization of metastases in our model of rapid recurrence, and also evaluate lesions growth rates to a high degree of accuracy over time. This project focuses on the kinetics of metastatic outgrowth both with and without surgical intervention, as a means of characterizing rapid metastatic recurrence further.
"Don't Mess With The Pancreas"
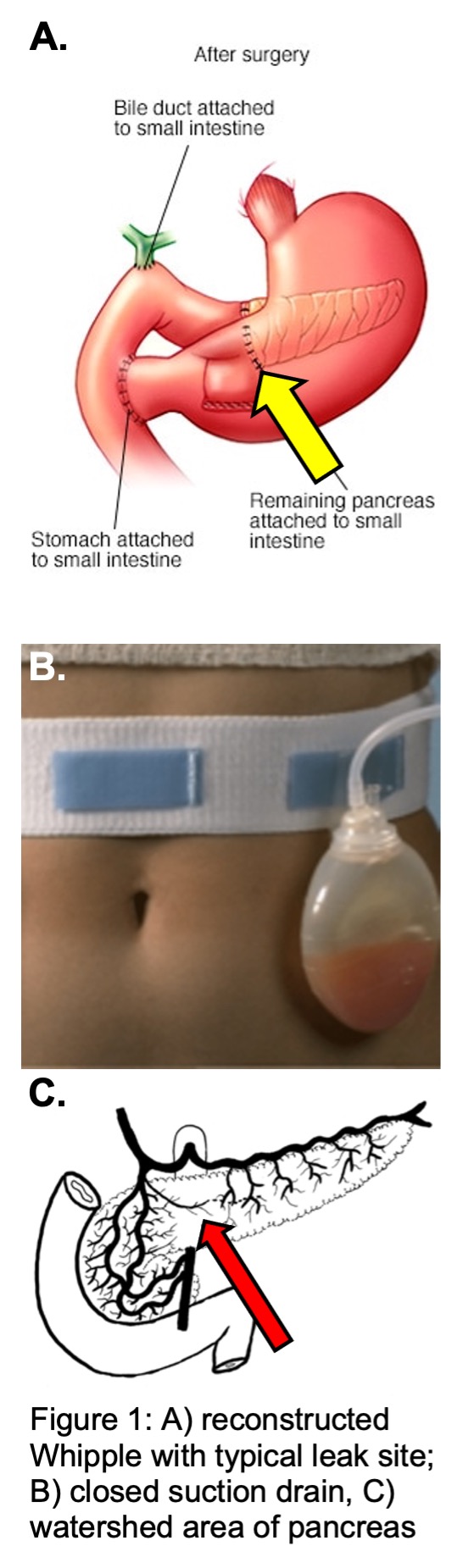
This surgical aphorism stems from the difficulty we have working with a gland that digests the very things that we are made of. In pancreatic surgery for removing the head of the pancreas (Whipple procedure), re-plumbing the pancreas so that digestive juices make their way into the digestive tract is critical, but highly prone to leaks which range from insignificant to devastating. These leaks occur in 25-40% of patients after pancreatic surgery and can prolong recovery, drive other causes of morbidity, and can delay chemotherapy in patients with cancer.
The area where the pancreas is typically divided is a "watershed zone" between the head of the pancreas, supplied by the gastroduodenal artery, and the tail of the pancreas, supplied by branches of the splenic artery. We hypothesize that this watershed zone becomes ischemic at transection, and that demonstrating hypoperfusion in this portion of the gland will associate with leaks. The Worth Lab is funded to study hypoperfusion at the transected margin using a safe, inexpensive, and readily-available FDA-approved technique for quantifying perfusion at this margin. We are planning to move this work into a multicenter study, and eventually translate this into a prospective trial randomizing hypoperfused patients to re-resection of the margin to better perfused tissue.