Cancer Immunotherapy
Yantasee Lab in collaboration with PDX Pharmaceuticals, Inc. employs novel nanotechnology to develop next-generation combination immunotherapies for cancer. Our proprietary Pdx-NP™ is innovative silica-based drug delivery technology that can load multiple cargo types at once (e.g., oligonucleotides, adjuvants, antibodies, chemotherapeutics, proteins) with superior outcomes than the free drug counterparts, creating a myriad of new intellectual properties. It can target both cancer and immune cells, generating cancer specific immune response.
In Situ Cancer Vaccine
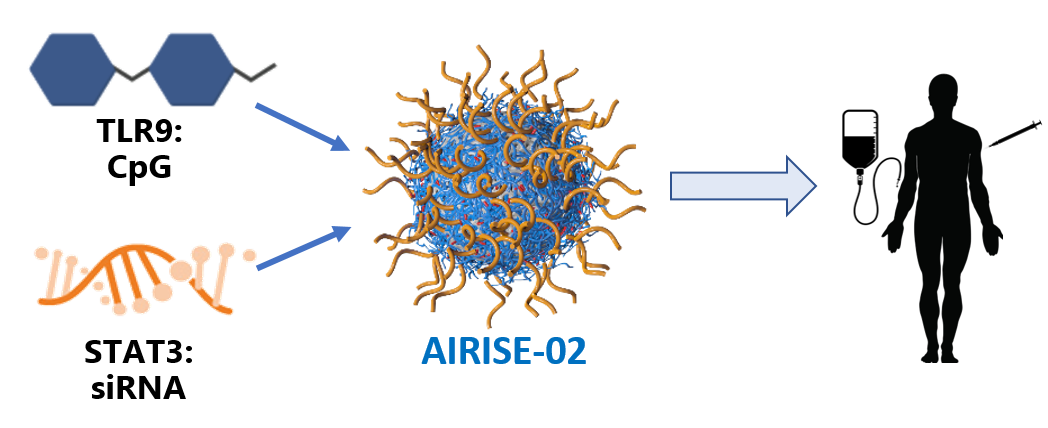
AIRISE (Augmenting Immune Response and Inhibiting Suppressive Environment of tumors) is our therapeutic cancer vaccine pipeline that utilize patients’ own tumors as depot to generate whole-body antitumor immune effects. AIRISE consists of nanoparticles co-delivering agents that stimulate immune response and inhibit immunosuppressive pathways. AIRISE administered to local tumors (e.g., melanoma, lung cancer, breast cancer, colorectal cancer) successfully primed adaptive immune system that controls growth of both local and distal tumors (e.g. metastasis). AIRISE circumvents the need to identify and generate neoantigens used in classical cancer vaccines. These neoantigens vary widely across patients, making it difficult and costly to develop personalized vaccines. AIRISE utilizes off-the-shelf drugs to create personalized anti-tumor immune effects.
In-situ tumor vaccination triggers adaptive immune response.
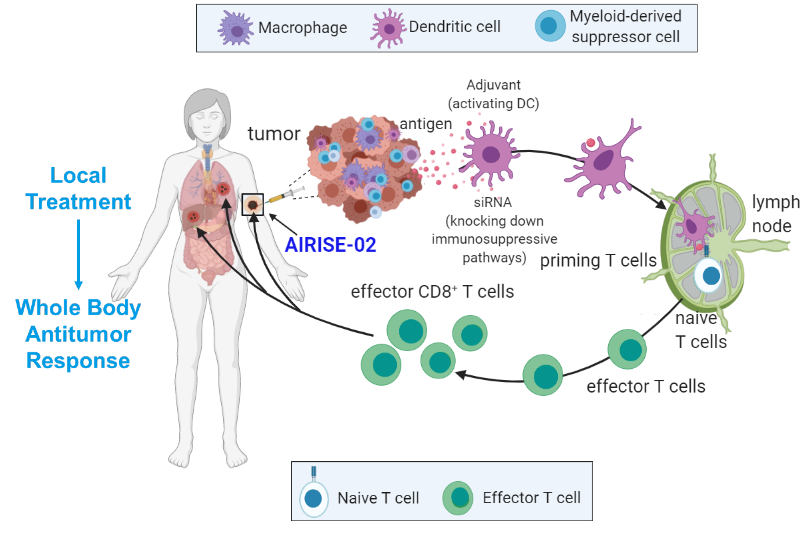
AIRISE utilizes a local treated tumor as a depot for neo-antigens in situ to trigger adaptive immunity (cytotoxic T cells) that kills tumors everywhere in the body.
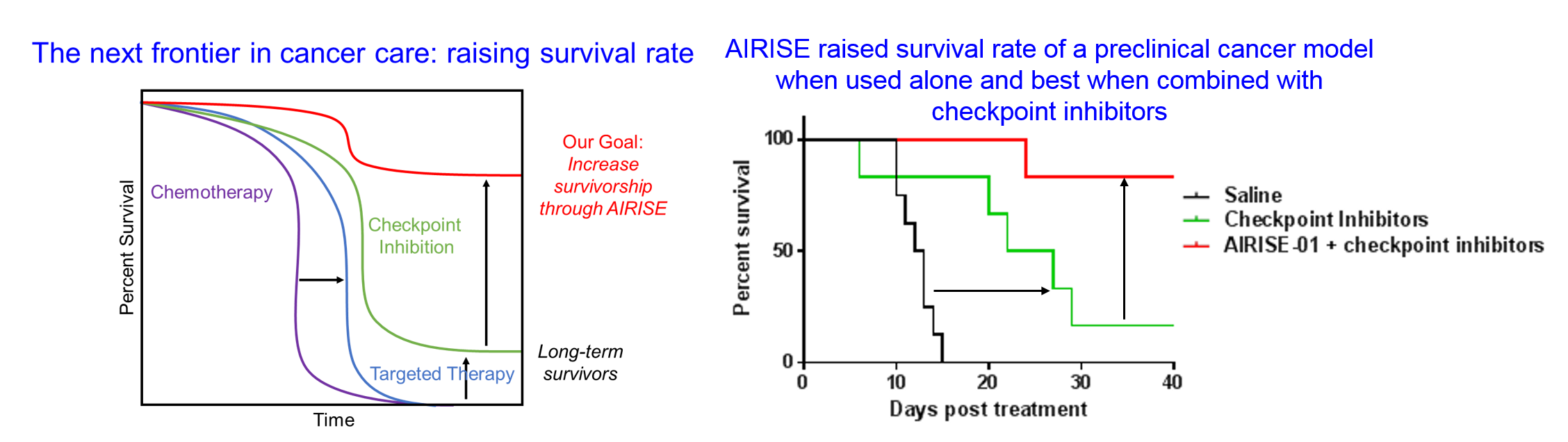
AIRISE improves the effect of checkpoint inhibitors and raises survival rate.
Only 3 doses of AIRISE are effective in a bilateral melanoma mouse model.
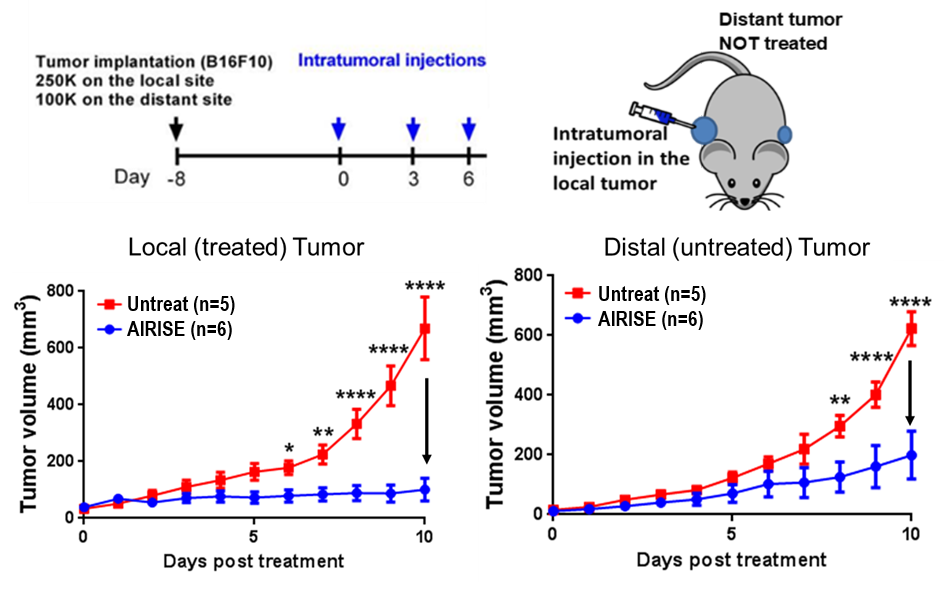
Both tumors were substantially reduced; owing to CD8+ T cell response (confirmed by CD8 depletion).
Mice were cured and were tumor-free for > 17 months even after tumor rechallenge, indicating memory T cell effect.
AIRISE-02 publication was featured as a cover article in Advanced Materials (IF 30.8).
Publication: Ngamcherdtrakul, W., Reda, M., Nelson, M. A., Wang, R., Zaidan, H. Y., Bejan, D. S., Hoang, N. H., Lane, R. S., Luoh, S.-W., Leachman, S. A., Mills, G. B., Gray, J. W., Lund, A. W., Yantasee, W., In Situ Tumor Vaccination with Nanoparticle Co-Delivering CpG and STAT3 siRNA to Effectively Induce Whole-Body Antitumor Immune Response. Adv. Mater. 2021, 2100628. | Read Online
Funding: National Cancer Institute R44CA265751, 2021-2024
Systemic Next Generation Cancer Immunotherapy for Broad Cancer Types
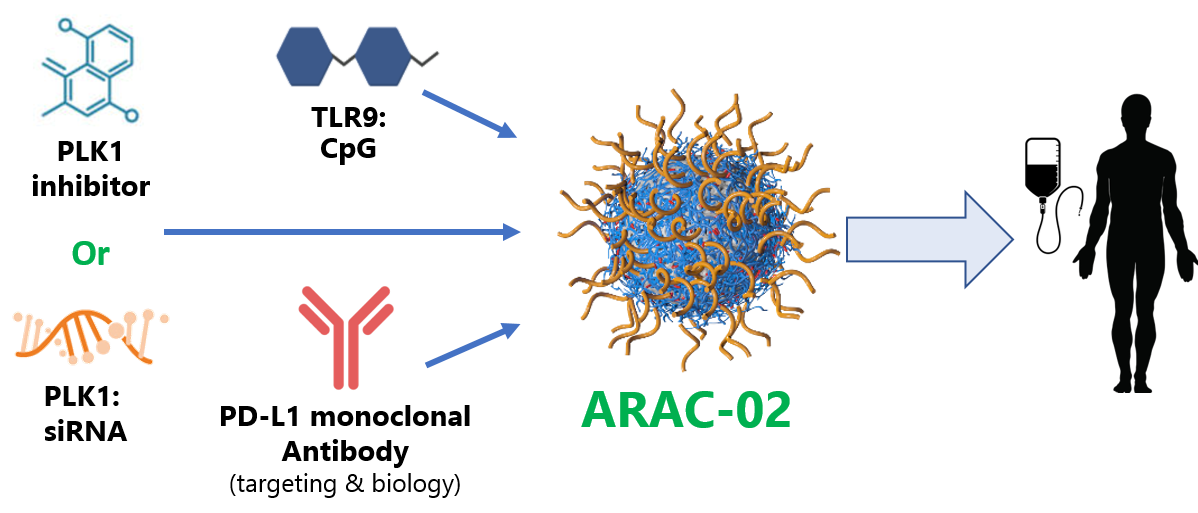
ARAC (Antigen Release Agent and Checkpoint inhibitor) is a systemic nano-immunotherapy for tumors inaccessible for local treatment or injection. ARAC consists of nanoparticle co-delivering a kinase inhibitor and immunotherapy to kill cancer cells and unleash the power of the immune system.
ARAC-02 deploys various complementary strategies to invoke pronounced immune responses:
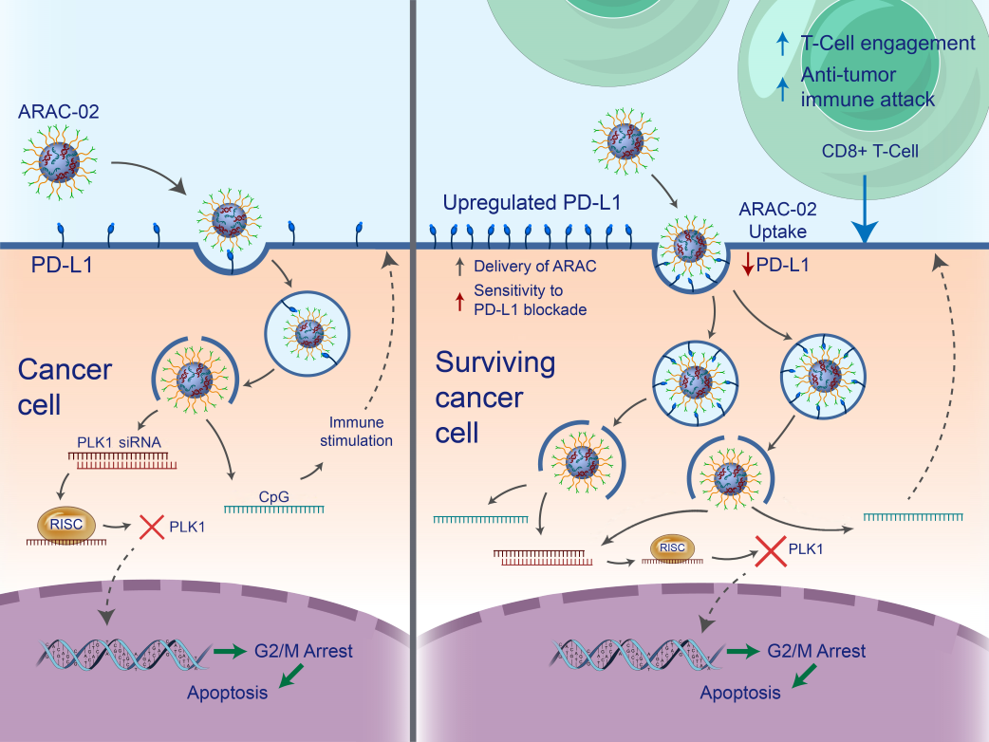
- PLK1 kills cancer cells, upregulating PD-L1 in surviving cells
- Facilitates additional tumor targeting via PD-L1 antibody
- Sensitizes tumors to PD-L1 inhibition
- CpG further primes immune system
- Cancer killing CD8+ T cells are generated, further amplifying the response
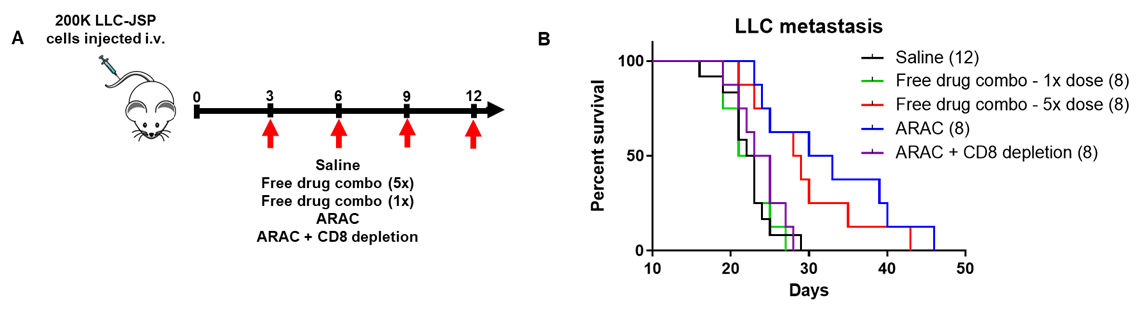
ARAC reduces the effective dose of drugs by 5-fold in a metastatic lung tumor model and the effect is immune (CD8+ T) mediated.
ARAC reduces tumor growth and prolongs survival in ICI-refractory KLN-205 syngeneic tumor model.
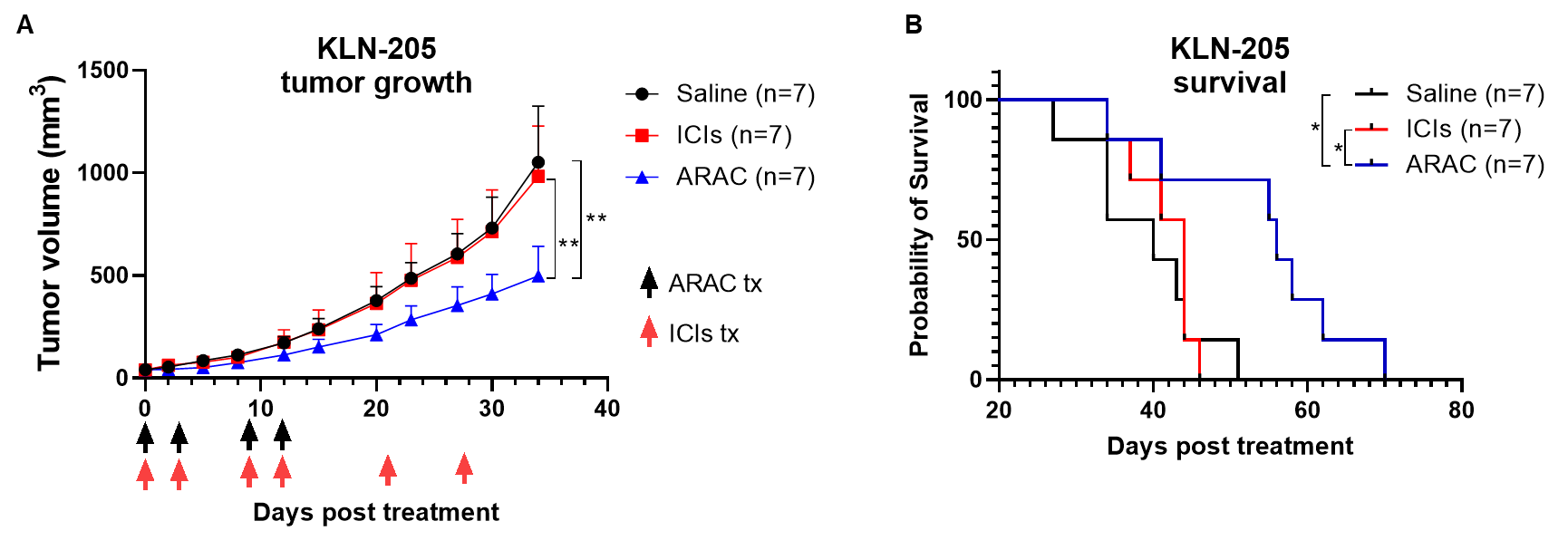
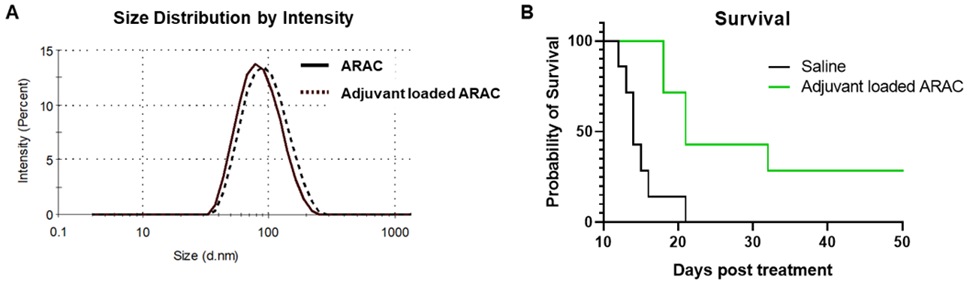
Adjuvant loaded ARAC-02 leads to cure of lung cancer in mice.
ARAC-02 treatment significantly improves survival of lung tumor (LLC-JSP) mice as compared to individual components and ARAC (without adjuvant).
Publication: Reda, M., Ngamcherdtrakul, W., Nelson, M. A., Siriwon, N., Wang, R., Zaidan, H. Y., Bejan, D. S., Reda, S., Hoang, N. H., Crumrine, N. A., Rehwaldt, J. P.C., Bindal, A., Mills, G. B., Gray, J. W., Yantasee, W., Development of novel nanoparticle-based immunotherapy targeting PD-L1 and PLK1 for lung cancer treatment. Nat. Commun. In press.
ARAC technology received a perfect score at NIH and is now funded by the National Cancer Institute (R44CA265752), 2021-2023