Projects
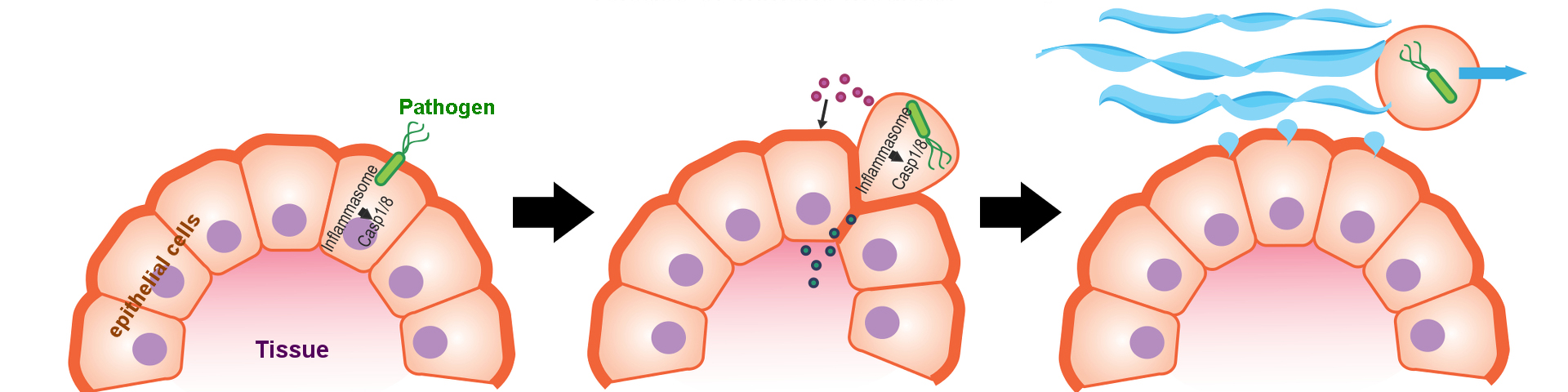
Intestinal epithelial cells act as a first barrier against pathogen intrusion. One effective way to prevent traversal of invading bacteria to the underlying tissue is inflammasome-induced epithelial cell extrusion of infected epithelial cells, a process we and others described recently. Inflammasomes are cytosolic sensors of pathogenic behavior, allowing cells to distinguish between harmless commensals and pathogens, which try to access the cell. After an inflammasome gets activated, cleavage of Caspases is triggered, which leads to a cascade of downstream events such as lytic cell death and cytokine release. We still don’t understand many aspects of this fascinating cell biological process, especially in epithelial cells. Using stem cell derived organoid in vitro as well as in vivo models of gastrointestinal pathogen infection for our research, we are currently aiming to answer the following questions:
- What are the signals downstream of inflammasome activation that lead to epithelial cell extrusion? (Collins Medical Trust funded)
- Which inflammasome sensors do intestinal epithelial cells use, is there mouse- human differences (NIH F32 funded)?
- Are certain pathogens capable of suppressing this response with effector molecules? What are the consequences?
- What is the role of inflammasomes in tuft cells, a specialized type of epithelial cell? (NIH funded R01)
If you think these are exciting questions to study, or want to discuss the many other project ideas we (or you?) have, contact us.