Members and Affiliates
Members
Global Child Health Research Focus

Despite widespread use of BCG, the only tuberculosis (TB) vaccine, TB remains a major cause of childhood morbidity and mortality worldwide. An improved childhood TB vaccine is urgently needed and requires an in depth understanding of the infant TB immunity so that TB vaccines can be developed that harness the limited potential of the infant immune system.
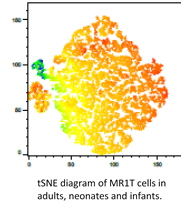
Towards this end, with their collaborators at the South African Tuberculosis Vaccine Initiative (SATVI, Dr. Thomas Scriba, Laboratory Director), Deborah Lewinsohn's laboratory is studying the ability of BCG and new TB vaccines to induce TB immunity across the human life span. The lab is particularly interested in studying MR1-restricted T cells (MR1T cells), which are an innate T cell present in young children. As MR1Ts sample the microbial metabolome, they serve a unique niche in the immune response to infectious diseases. The Deborah Lewinsohn lab is studying the influence of BCG vaccination on MR1T cell immunity with the goal of understanding how BCG and other TB vaccines may utilize MR1T cells to effect protection against childhood TB.
WEBSITE | Deborah Lewinsohn's Lab
WEBSITE | Oregon Tuberculosis Research Lab (OTBRL)
Relevant Publications
-
Polyfunctional CD4+ T cells as targets for tuberculosis vaccination. / Lewinsohn, Deborah; Lewinsohn, David; Scriba, Thomas J.
In: Frontiers in Immunology, Vol. 8, No. OCT, 1262, 05.10.2017.
-
MR1-restricted MAIT cells display ligand discrimination and pathogen selectivity through distinct T cell receptor usage. / Gold, Marielle; McLaren, James E.; Reistetter, Joseph A.; Smyk-Pearson, Sue; Ladell, Kristin; Swarbrick, Gwendolyn M.; Yu, Yik Y L; Hansen, Ted H.; Lund, Ole; Nielsen, Morten; Gerritsen, Bram; Kesmir, Can; Miles, John J.; Lewinsohn, Deborah; Price, David A.; Lewinsohn, David.
In: Journal of Experimental Medicine, Vol. 211, No. 8, 2014, p. 1601-1610.
- Gold, M., Eid, T., Smyk-Pearson, S., Eberling, Y., Swarbrick, G. M., Langley, S. M., ... Lewinsohn, D. (2013). Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. Mucosal
Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. / Gold, Marielle; Eid, T.; Smyk-Pearson, S.; Eberling, Y.; Swarbrick, G. M.; Langley, S. M.; Streeter, Philip; Lewinsohn, Deborah; Lewinsohn, David.
In: Mucosal Immunology, Vol. 6, No. 1, 01.2013, p. 35-44.

Global Child Health Research Focus

The David Lewinsohn laboratory endeavours to understand the mechanisms by which the human immune system recognizes those cells infected with Mycobacterium tuberculosis (Mtb). In this regard, they have focused on CD8+ T cells.
Projects in the lab run the full gamut of the analysis of the mechanisms by which Mtb antigens gain access to the processing and presentation pathways (Cell Biology); to defining the classical and non-classical HLA molecules required for the display of these ligands (Cellular Immunology); to understanding the role of these cells in those infected with Mtb (Translational Immunology). As Mtb is an intracellular infection, Dr. Lewinsohn has focused his work on the premise that it is important for the immune system to identify and respond to those cells that harbor the pathogen. Human, Mtb-specific CD8 T cells are present at high frequency in those infected with Mtb, and may serve as a surrogate for the degree of intracellular infection.
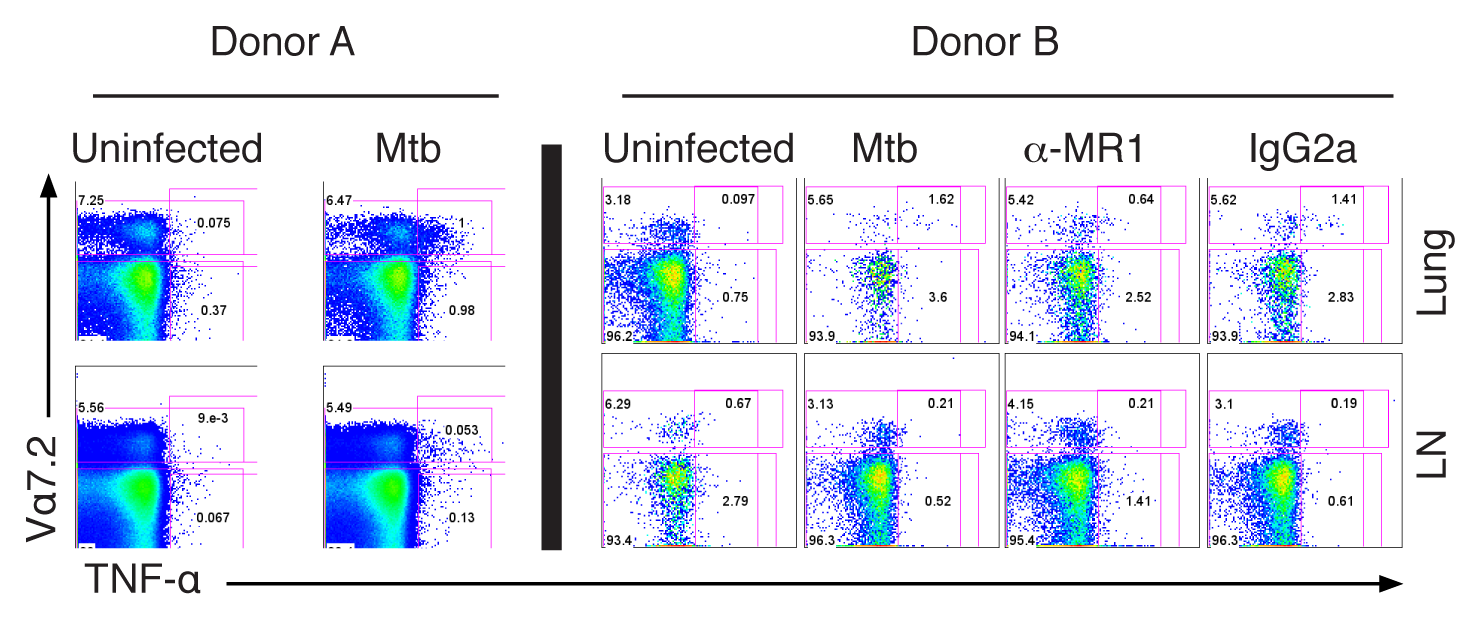
In order to define those antigens that are both commonly and robustly recognized, the David Lewinsohn lab has screened to peptide libraries for CD8 responses directly ex-vivo. This analysis was done using ethnically diverse donors, and immune-dominant antigens validated in both adult and pediatric populations in collaboration with Case Western Reserve University (Henry Boom) and Makerere University (Kampala, Uganda; Harriett Myanja and Sarah Kiguli). Most recently, the Bill and Melinda Gates Foundation has funded the lab to explore the hypothesis that a special family of human T cells—Mucosal Associated Invariant T (MAIT) cells—can be harnessed as a vaccine to prevent TB.
WEBSITE | David Lewinsohn's Lab
WEBSITE | Oregon Tuberculosis Research Lab (OTBRL)
Relevant Publications
-
Characterization of specific CD4 and CD8 T-cell responses in QuantiFERON TB Gold-Plus TB1 and TB2 tubes. / Allen, Nadia PC; Swarbrick, Gwendolyn; Cansler, Meghan; Null, Megan; Salim, Hiam; Miyamasu, Misato; Howard, Jenny; Boyle, Jeffrey; Lewinsohn, David; Lewinsohn, Deborah.
In: Tuberculosis, Vol. 113, 01.12.2018, p. 239-241.
-
Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. / Gold, Marielle; Eid, T.; Smyk-Pearson, S.; Eberling, Y.; Swarbrick, G. M.; Langley, S. M.; Streeter, Philip; Lewinsohn, Deborah; Lewinsohn, David.
In: Mucosal Immunology, Vol. 6, No. 1, 01.2013, p. 35-44.
-
Human mucosal associated invariant T cells detect bacterially infected cells. / Gold, Marielle; Cerri, Stefania; Smyk-Pearson, Susan; Cansler, Meghan E.; Vogt, Todd M.; Delepine, Jacob; Winata, Ervina; Swarbrick, Gwendolyn M.; Chua, Wei Jen; Yu, Yik Y L; Lantz, Olivier; Cook, Matthew S.; Null, Megan D.; Jacoby, David; Harriff, Melanie; Lewinsohn, Deborah; Hansen, Ted H.; Lewinsohn, David.
In: PLoS Biology, Vol. 8, No. 6, 06.2010.
Global Child Health Research Focus

B. Alex Foster, MD, MPH is a pediatrician-scientist. His global health research focuses on children living with HIV and how to improve their clinical outcomes and quality of life. This work is done primarily in partnership with faculty at Hawassa University in Ethiopia. His recent work, in partnership with Dr. Birkneh Tadesse, has been on identifying predictors of treatment failure in children infected with HIV, using a large cohort of children and implementing viral load testing as a more sensitive indicator of treatment failure. His other research interests address obesity in childhood, particularly for low-income populations at higher risk of continued obesity and the subsequent associated complications.
Dr. Foster attended medical and graduate school at OHSU, completed residency training in New York City, and then started his faculty career in Texas before returning to Oregon. He holds faculty appointments in both the School of Medicine and the OHSU-PSU School of Public Health.
Relevant Publications
- Hepatic and renal toxicity and associated factors among HIV-infected children on antiretroviral therapy : a prospective cohort study. / Tadesse, B. T.; Foster, Byron (Alex); Kabeta, A.; Ayalew, F.; H/Meskel, G.; Jerene, D.; Makonnen, E.; Aklillu, E. In: HIV Medicine, Vol. 20, No. 2, 01.02.2019, p. 147-156.
- High levels of dual-class drug resistance in HIV-infected children failing first-line antiretroviral therapy in Southern Ethiopia. / Tadesse, Birkneh Tilahun; Kinloch, Natalie N.; Baraki, Bemuluyigza; Lapointe, Hope R.; Cobarrubias, Kyle D.; Brockman, Mark A.; Brumme, Chanson J.; Foster, Byron (Alex); Jerene, Degu; Makonnen, Eyasu; Aklillu, Eleni; Brumme, Zabrina L.In: Viruses, Vol. 10, No. 2, 60, 01.02.2018.
- Cohort profile : Improving treatment of HIV-infected Ethiopian children through better detection of treatment failure in southern Ethiopia. / Tadesse, Birkneh Tilahun; Foster, Byron (Alex); Jerene, Degu; Ruff, Andrea. In: BMJ open, Vol. 7, No. 2, e013528, 01.02.2017.
Global Child Health Research Focus

Dr. Christina Lancioni attended medical school at the University of Pennsylvania, and went on to complete residency training in Pediatrics at Children’s National Medical Center in Washington, DC, in 2004. During this time, Dr. Lancioni volunteered for a non-for-profit organization that provides subspecialty medical education to providers in low and middle income countries, and was able to teach Pediatric HIV-care to providers in Uganda, Malawi, and Botswana. While completing her fellowship training in Pediatric Infectious Diseases at Case Western Reserve University in Cleveland, OH, from 2006- 2009, Dr. Lancioni established collaborations with physician-scientists based at Makerere University, in Kampala, Uganda. Working together with her Ugandan colleagues, Dr. Lancioni has since gone on to study interactions between TB and the immune system, the burden of TB in children, and the impact of undernutrition on childhood survival following acute illness.
In partnership with her long-time colleague Dr. Ezekiel Mupere (currently Chair of Pediatrics at Makerere University), Dr. Lancioni serves as Co-Principle Investigator for the Uganda site of the “Childhood Acute Illness & Nutrition” Network, or CHAIN (see chainnetwork.org). The CHAIN network is a group of investigators working in 6 countries in sub-Saharan and SE Asia (Uganda, Kenya, Malawi, Burkina Faso, Pakistan, Bangladesh) to identify biological mechanisms and socio-economic factors that determine a child’s risk of death or serious morbidity during acute hospitalization, and for the 6 months following a hospital admission. In addition to her role as co-PI, Dr. Lancioni leads studies to delineate the burden of TB among young, acutely ill children, and to understand immunologic perturbations that may contribute to poor outcomes from acute illness during childhood. Additional interests of the CHAIN Network that are supported by the Ugandan CHAIN team, are to understand the impact of acute illness and undernutrition on childhood neurodevelopment, and to understand how differences in body composition contribute to growth and recovery from acute illness during early childhood. The CHAIN Network is funded by the Bill & Melinda Gates Foundation.
Relevant Publications

- Gwela A, Mupere E, Berkley JA, Lancioni C. Undernutrition, Host Immunity, and Vulnerability to Infection among Young Children. The Pediatric Infectious Disease Journal. 2019. In Press.
-
Lancioni C, Swarbrick GM, Park B, Nyendak M, Nsereko M, Mayanja-Kizza H, Null MD, Cansler ME, Duncan A, Baseke J, Chervenak K, Malone L, Heaphy EG, Boom WH, Lewinsohn DM, Lewinsohn DA. Recognition of CD8+ T cell Epitopes to Identify Adults with Pulmonary Tuberculosis. Eur Resp J. 2019. In Press.
-
Johnson, D. F., Malone, L. L., Zalwango, S., Oketcho, J. M., Chervenak, K. A., Thiel, B., ... Lancioni, C. (2014). Tuberculin skin test reversion following isoniazid preventive therapy reflects diversity of immune response to primary Mycobacterium tuberculosis infection. PLoS One, 9(5), [e96613]. https://doi.org/10.1371/journal.pone.0096613
-
Jaganath, D., Zalwango, S., Okware, B., Nsereko, M., Kisingo, H., Malone, L., ... Mupere, E. (2013). Contact investigation for active tuberculosis among child contacts in Uganda. Clinical Infectious Diseases, 57(12), 1685-1692. https://doi.org/10.1093/cid/cit645
Research Focus

Dr. McEvoy's long term research goal is to advance the understanding, prevention, and treatment of the perinatal origins of neonatal and infant lung disease. She is approaching this goal through clinical and translational projects that incorporate the use of pulmonary function testing (PFTs). My early publications investigated the effect of shorter and lower doses of dexamethasone on PFTs than had historically been given to premature babies at risk for bronchopulmonary dysplasia. These publications demonstrated that the measurement of newborn and infant PFTs can be reproducibly performed, can quantify the impact of perinatal factors on newborn and infant lung function, and can correlate with clinical outcomes, therefore allowing the study of a smaller sample size than would be needed for a pure clinical outcome. This work also demonstrated that lower total doses of dexamethasone resulted in the same improvements in PFTs as higher doses, thus decreasing the potential of adverse effects from therapy.
The McEvoy lab is currently studying the ability of vitamin C supplementation during pregnancy to block the effects of in utero smoke on newborn and infant PFTs. Since at least 10% of women continue to smoke during pregnancy, vitamin C may be a simple, safe and inexpensive way to decrease the effects of smoking in pregnancy on infant pulmonary function and respiratory morbidities.
Relevant Publications
-
Candida albicans-associated sepsis in a pre-term neonatal rhesus macaque (Macaca mulatta). / Pecoraro, Heidi L.; Berg, Melissa R.; Dozier, Brandy; Martin, Lauren Drew; McEvoy, Cynthia (Cindy); Davies, Michael H.; Ducore, Rebecca.
In: Journal of medical primatology, 01.01.2019.
-
Pulmonary Effects of Maternal Smoking on the Fetus and Child : Effects on Lung Development, Respiratory Morbidities, and Life Long Lung Health. / McEvoy, Cynthia (Cindy); Spindel, Eliot.
In: Paediatric Respiratory Reviews, 2016.
-
Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants : A randomized clinical trial. / McEvoy, Cynthia (Cindy); Schilling, Diane; Clay, Nakia; Jackson, Keith; Go, Mitzi D.; Spitale, Patricia; Bunten, Carol; Leiva, Maria; Gonzales, David; Hollister-Smith, Julie; Durand, Manuel; Frei, Balz; Buist, A (Sonia); Peters, Dawn; Morris, Cynthia; Spindel, Eliot.
In: JAMA - Journal of the American Medical Association, Vol. 311, No. 20, 2014, p. 2074-2082.
Global Child Health Research Focus

Infectious diarrheal diseases, caused by a variety of bacterial and viral pathogens, remains unfortunately prevalent in many parts of the world where provision of safe water supply and reliable sanitation has yet to be achieved. Persistent diarrhea places affected children as risk for growth stunting, which is strongly associated globally with reduced intellectual achievement, both individually and at a population level. As part of an effort to define possible approaches to reduce the burden of diarrhea in children, Dr. Scottoline’s laboratory is investigating the fate of immunoglobulin (Ig)—those in breast milk, or exogenous Ig, in the infant gastrointestinal tract. This work is in collaboration with Dr. Dave Dallas’ laboratory at Oregon State University, and is being conducted in conjunction with efforts to develop monoclonal antibodies (MAbs) which are targeted against organisms that cause infectious diarrhea, and are to be given via an enteral route.
Healthy growth in malnourished children can be surprisingly difficult to achieve, and there essentially no tools, aside from anthropomorphics, to assess healthy growth in children that are economical and high volume. Dr. Scottoline’s lab has validated a safe, inexpensive, easy to use, and accurate means to measure skeletal muscle and fat free mass using muscle biochemistry and heavy isotope labeling. Dr. Scottoline plans to use this newly developed tool to define normative values for infant skeletal muscle mass and growth, and to explore the utility of this tool in the growth of very preterm infants, in whom extra-uterine growth restriction is a vexing challenge, as a model of lean mass accrual in malnourished children.
Relevant Publications
-
Neonatal McCune–Albright syndrome with survival beyond two years. / Pierce, Melinda; Scottoline, Brian.
In: American Journal of Medical Genetics, Part A, Vol. 170, No. 11, 01.11.2016, p. 3008-3012.
-
Steroid-induced resolution of refractory pulmonary interstitial emphysema. / Mahapatra, Sidharth; Scottoline, Brian.
In: Journal of Maternal-Fetal and Neonatal Medicine, 28.03.2016, p. 1-4.
-
Long-term survival with diaphanospondylodysostosis (DSD) : Survival to 5 years and further phenotypic characteristics. / Scottoline, Brian; Rosenthal, Scott; Keisari, Rami; Kirpekar, Rashmi; Angell, Cathy; Wallerstein, Robert.
In: American Journal of Medical Genetics, Part A, Vol. 158 A, No. 6, 06.2012, p. 1447-1451.
Affiliates
Infectious Diseases
Global Child Health Research Focus

The international community continues to face substantial global health challenges posed by infectious diseases including many established viral illnesses such as HIV and dengue, and emerging viruses such as Zika. Despite ongoing research efforts, these illnesses continue to cause substantial morbidity and mortality worldwide due to critical hurdles along the continuum from the laboratory bench to the implementation of effective public health measures capable of treating and preventing illness.
The Curlin laboratory is working on many fronts to improve its capacity to diagnose viral infections; understand the key pathophysiological and host defense mechanisms shaping disease expression; and develop effective treatment and disease prevention modalities. Their current efforts are focused on these issues as they relate to Southeast Asia, and include laboratory research, translational and behavioral clinical research, and epidemiological methods. One important dimension of the lab's international work is to foster the development of in loco early-career investigators committed to these important areas of biomedical research
Relevant Publications:
- Association between HIV genotype, viral load and disease progression in a cohort of Thai men who have sex with men with estimated dates of HIV infection. / Leelawiwat, Wanna; Pattanasin, Sarika; Sriporn, Anuwat; Wasinrapee, Punneeporn; Kongpechsatit, Oranuch; Mueanpai, Famui; Tongtoyai, Jaray; Holtz, Timothy H.; Curlin, Marcel. In: PLoS One, Vol. 13, No. 7, e0201386, 01.07.2018.
- Daily and Nondaily Oral Preexposure Prophylaxis in Men and Transgender Women Who Have Sex with Men : The Human Immunodeficiency Virus Prevention Trials Network 067/ADAPT Study. / Grant, Robert M.; Mannheimer, Sharon; Hughes, James P.; Hirsch-Moverman, Yael; Loquere, Avelino; Chitwarakorn, Anupong; Curlin, Marcel E.; Li, Maoji; Amico, K. Rivet; Hendrix, Craig; Anderson, Peter L.; Dye, Bonnie J.; Marzinke, Mark A; Piwowar-Manning, Estelle; McKinstry, Laura; Elharrar, Vanessa; Stirratt, Michael; Rooney, James F.; Eshleman, Susan; McNicholl, Janet M.; Van Griensven, Frits; Holtz, Timothy H. In: Clinical Infectious Diseases, Vol. 66, No. 11, 17.05.2018, p. 1712-1721.
- Analysis of false-negative human immunodeficiency virus rapid tests performed on oral fluid in 3 international clinical research studies. / Curlin, Marcel; Gvetadze, Roman; Leelawiwat, Wanna; Martin, Michael; Rose, Charles; Niska, Richard W.; Segolodi, Tebogo M.; Choopanya, Kachit; Tongtoyai, Jaray; Holtz, Timothy H.; Samandari, Taraz; Mcnicholl, Janet M. In: Clinical Infectious Diseases, Vol. 64, No. 12, 15.06.2017, p. 1663-1669.
Global Child Health Research Focus

The Messer lab's research has focused on dengue virus, a pathogen transmitted by mosquitos and endemic throughout the tropical and sub-tropical world. With the recent emergence of Zika virus in the Americas, Dr. Messer has expanded his research to include Zika, as Zika and dengue are closely related. The lab's particular interest is in viral genetics and evolution and how those factors affect viral fitness, viral pathogenicity, and the human immune response to dengue and Zika virus infection over time.
To study this interplay, the lab has a cohort of dengue and Zika immune human subjects who live in non-endemic Portland, Oregon. Using immune sera and cells collected longitudinally from this cohort and a panel of 60 clinical virus isolates, they study the natural history of dengue and Zika immunity over time, unconfounded by repeat infections.
The Messer lab also characterizes—at a single cell level—the dengue and Zika virus epitopes that are recognized by human memory B-cells and the potency and breadth of the antibodies these memory B-cells are programmed to secrete. Their work informs both understanding of the determinants of antibody mediated protection from flavivirus infection and rationale dengue and Zika vaccine design.
Relevant Publications
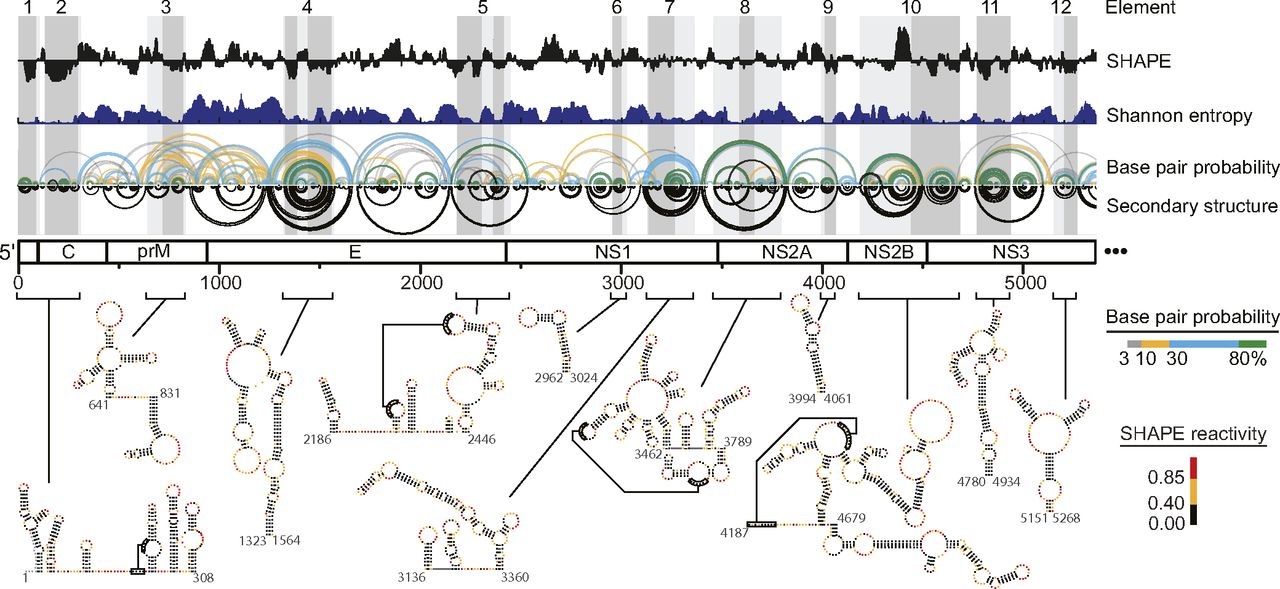
- Dethoff EA, Boerneke MA, Gokhale NS, et al. Pervasive tertiary structure in the dengue virus RNA genome. Proc Natl Acad Sci U S A. 2018;115(45):11513–11518. doi:10.1073/pnas.1716689115
- Leier HC, Messer WB, Tafesse FG. Lipids and pathogenic flaviviruses: An intimate union. PLoS Pathog. 2018;14(5):e1006952. Published 2018 May 10. doi:10.1371/journal.ppat.1006952
- Messer WB, Yount BL, Royal SR, et al. Functional Transplant of a Dengue Virus Serotype 3 (DENV3)-Specific Human Monoclonal Antibody Epitope into DENV1. J Virol. 2016;90(10):5090–5097. Published 2016 Apr 29. doi:10.1128/JVI.00155-16
Molecular Microbiology and Immunology
Global Child Health Research Focus

The goals of the Purdy laboratory are to further define the intrinsic resistance of the pathogen to the host immune response and antibiotics, delineate pathways in mycobacterial cell wall biogenesis, and identify targets and new strategies for future drug therapy. The lab combines the approaches of bacterial genetics, biochemistry and cell biology to achieve these goals.
Current efforts in the Purdy lab are focused on characterizing the function and regulation of mycobacterial MmpL cell wall lipid transporters that are crucial contributors to mycobacterial physiology and pathogenesis. Their focus is on MmpL11, which is conserved in non-pathogenic and pathogenic mycobacteria. MmpL11 plays a conserved role in mycobacterial biofilm formation. They have identified the lipids transported by MmpL11 as monomeromycolyl diacylglycerol (MMDAG), long chain TAGs, and mycolate wax ester (MWE). These lipids have been classified as “storage lipids” and are implicated in non-replicating persistence or latency. Consistent with this role, the Purdy lab showed that the M. tuberculosis mmpL11 mutant is impaired for survival and/or resuscitation compared to wild-type M. tuberculosis when incubated under nutrient and oxygen starvation. Furthermore, they showed that the mmpL11 mutant is less fit than wild-type in an in vitro granuloma model. Combined these data suggest that MmpL11 and its substrates are important for M. tuberculosis non-replicating persistence and latency.
The long-term impact of pursuing this research is significant because the nature of non-replicating Mtb persistence and the transitions between a highly replicative state to latent tuberculosis infection are important, but poorly understood, aspects of Mtb pathogenesis. Non-replicating persistence is particularly relevant to the clinic because these Mtb exhibit phenotypic drug tolerance. As such, we and others are keen to identify compounds that target these quiescent organisms (Foss et al., 2016). I have a demonstrated track record of using genetic and biochemical techniques to identify and structurally characterize cell envelope lipids that contribute to this phenotype (Wright, et al., 2017; Pacheco et al., JBC 2013; Purdy et al., 2013).
Relevant Publications
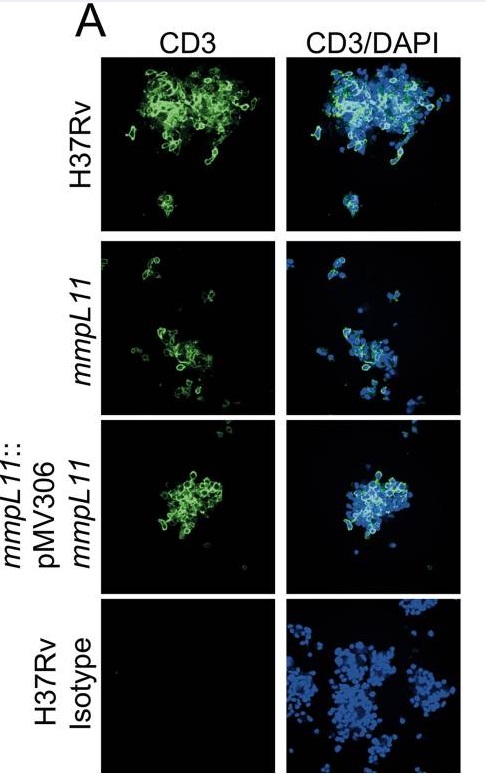
- Wright, C.C., Hsu, F.F. Arnett, E., Dunaj, J.L, Davidson, P.M., Pacheco, S.A., Harriff, M.J., Lewinsohn, D.M., Schlesinger, L.S., and G.E. Purdy. 2017. The Mycobacterium tuberculosis MmpL11 Cell Wall Lipid Transporter Is Important for Biofilm Formation, Intracellular Growth, and Nonreplicating Persistence. Infect Immun 85: e00131–17. PMID: 28507063 PMC5520431
- Foss, M.H., Pou, S., Davidson, P.M., Dunaj, J., Winter, R.W., Pou, S., Licon, M.H., Doh, J., Li, Y., Kelly, J., Dodean, R., Koop, D., Riscoe, M.K. and G.E. Purdy. 2016 Diphenylether-modified 1,2-diamines with improved drug properties for development against Mycobacterium tuberculosis. ACS Infect Dis. 2(7):500-8. PMID: 27626102.
- Delmar, J.A. Chou, T-H, Wright, C.C., Licon,, M.H., Doh, J.K., Radhakrishnan, A., Kumar, N., Lei, H-T., Bolla, J.R., Rajashankar, K.R., Su, C-C., Purdy, G.E. and E. W. Yu. 2015 Structural basis for the regulation of the MmpL transporters of Mycobacterium tuberculosis. J. Biol. Chem. 290: 28559–28574. PMID 26396194 PMC4653710.
- Pacheco, S.A., F.F. Hsu, K.M. Powers, and G.E. Purdy. 2013. The MmpL11 transporter contributes to mycobacterial cell wall biosynthesis and biofilm formation in M. smegmatis. J. Biol. Chem. 288:24213-22. PMCID: PMC3745366.
Research Focus

The profound success of pathogens such as Mycobacterium tuberculosis and HIV in causing disease depends on their ability to utilize the host’s cellular machinery for their own advantage in averting its immune system. Understanding these pathways or processes essential for the life cycle of these pathogens is crucial, as it represents potential targets for new drug strategies.
Dr. Tafesse’s laboratory focuses on identifying and characterizing the host factors that are used by pathogens to secure invasion, persistence and propagation. They are especially interested in studying the role of cellular lipids in bacterial and/or viral pathogenesis and their significance on innate and adaptive immunity. The lab employs genome-wide genetic screens, various lipidomic analysis techniques and state-of-the art microscopy and other biochemical tools to define the pathogenesis of M. tuberculosis and HIV.
Additionally, Tafesse aims to apply novel strategies, such as the use of single-domain antibodies/nanobodies not only to unravel the intricate relationships of these pathogens with the host but also to use it as a diagnostic and therapeutics tools.
Relevant Publications
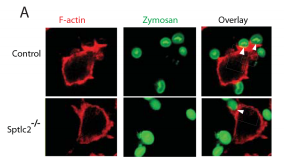
- Tafesse FG, Rashidfarrokhi A, Schmidt FI, Freinkman E, Dougan S, Dougan M, et al. (2015) Disruption of Sphingolipid Biosynthesis Blocks Phagocytosis of Candida albicans. PLoS Pathog 11(10): e1005188. doi:10.1371/journal.ppat.1005188
- Tafesse, F., Guimaraes, C. P., Maruyama, T., Carette, J. E., Lory, S., Brummelkamp, T. R., & Ploegh, H. L. (2014). GPR107, a G-protein-coupled receptor essential for intoxication by Pseudomonas aeruginosa exotoxin a, localizes to the Golgi and is cleaved by furin. Journal of Biological Chemistry, 289(35), 24005-24018. https://doi.org/10.1074/jbc.M114.589275
- Tafesse, F., Vacaru, A. M., Bosma, E. F., Hermansson, M., Jain, A., Hilderink, A., ... Holthuis, J. C. M. (2014). Sphingomyelin synthase-related protein SMSr is a suppressor of ceramide-induced mitochondrial apoptosis. Journal of Cell Science, 127(2), 445-454. https://doi.org/10.1242/jcs.138933
Nutrition
Global Child Health Research Focus

Diane Stadler, Ph.D., R.D., L.D. is actively engaged in OHSU’s global health initiatives both abroad and at home. She has worked in Zambia, Africa, with a focus on nutritional rehabilitation of severely malnourished children. She also volunteers with nonprofit organizations serving rural villages in eastern Honduras, where she trains community members to monitor growth of infants and young children at risk for malnutrition, to initiate nutritional rehabilitation and school-based meal programs, and to enhance maternal health.
Dr. Stadler’s Ph.D. is in Human Nutrition and she is a registered dietitian with expertise in maternal and infant nutrition. She completed a post-graduate fellowship at the Kennedy Krieger Institute & Johns Hopkins Hospital and specializes in the design and implementation of dietary interventions for children with congenital or acquired metabolic disorders or developmental disabilities. She studies the impact of diet on weight regulation and markers of disease risk and is a leader in OHSU’s nutrition education initiatives and research mentoring programs. Since 2008, Dr. Stadler has directed graduate programs in Human Nutrition at OHSU, which graduates 20 nutrition graduate students and dietetic interns each year.
Relevant Publications
- Environmental, dietary and case-control study of Nodding Syndrome in Uganda : A post-measles brain disorder triggered by malnutrition? / other Members of the Oregon-Uganda Nodding Syndrome Research Team. In: Journal of the Neurological Sciences, Vol. 369, 15.10.2016, p. 191-203.
- Calcium absorption in Nigerian children with rickets. / Graff, Mariaelisa; Thacher, Tom D.; Fischer, Philip R.; Stadler, Diane; Pam, Sunday D.; Pettifor, John M.; Isichei, Christian O.; Abrams, Steven A. In: The American journal of clinical nutrition, Vol. 80, No. 5, 11.2004, p. 1415-1421.
Neurology
Global Child Health Research Focus
While most neurodegenerative diseases are sporadic in occurrence, the environmental triggers are unknown and sparsely researched. Pockets of high-incidence disease provide unique opportunities to discover etiologies that can then be applied to the search for related disorders worldwide.
For example, the Spencer laboratory has a long-term research interest in seeking the environmental triggers of a prototypical neurodegenerative disease that has occurred in isolated populations in Guam, Japan and New Guinea. Ongoing studies focus on research in the field and laboratory, the latter involving mostly omics approaches to explore molecular perturbations induced by putative neurotoxic agents and their relationship to the human disease. While clinical disease is expressed in adults and the aged, critical environmental exposures occurred in early life.
Another example is Nodding syndrome, an enigmatic seizure disorder of children in certain parts of East Africa. Research underway with Ugandan colleagues explores the role of neuroviral and neurotoxic factors in this progressive neurodegenerative disorder.
Thirdly, collaborative research initiatives with China employ transgenic animals and advanced proteomics to explore risk factors for age-related neurodegenerative disorders.
Relevant Publications
- Spencer, P., & Kisby, G. (2019). Chemicals, somatic mutations and neurodegeneration: evidence from Western Pacific amyotrophic lateral sclerosis-parkinsonism-dementia complex (ALS-PDC): Commentary on: Leija-Salazar M, Piette C, Proukakis C. Review: Somatic mutations in neurodegeneration. Neuropathol Appl Neurobiol 2018; 44: 267–85. Neuropathology and Applied Neurobiology. https://doi.org/10.1111/nan.12533
- Valdes Angues, R., Suits, A., Palmer, V., Okot, C., Okot, R. A., Atonywalo, C., ... Spencer, P. (2018). A real-time medical cartography of epidemic disease (Nodding syndrome) using village-based lay mHealth reporters. PLoS Neglected Tropical Diseases, 12(6), [e0006588]. https://doi.org/10.1371/journal.pntd.0006588
- Spencer, P., Schmutzhard, E., & Winkler, A. S. (2017). Nodding Syndrome in the Spotlight – Placing Recent Findings in Perspective. Trends in Parasitology, 33(7), 490-492. https://doi.org/10.1016/j.pt.2017.05.001
Global Child Health Research Focus

A substantial body of evidence indicates that chronic dietary reliance of foodstuffs from insufficiently processed cassava have resulted in outbreaks of an irreversible paralysis of legs called konzo.
Konzo mainly affects children and women of childbearing age in several countries of sub-Saharan Africa, including Congo, Central African Republic, Cameroun, Angola, Mozambique, Uganda, Tanzania, and Zambia. The Thsala laboratory recently showed that subjects are likely to present with a spectrum of deficits ranging from subtle motor deficits to deficits in cognition. As konzo outbreaks increases, so does the number of countries impacted. When considered together, along with the recent evidence for cognition deficits in children from konzo areas, the overall burden of cassava neurological disease appears to have been underestimated. Risks for such disabilities are compounded by the potential for climate change to affect cassava cyanogenesis and yield high-cyanogen content cassava varieties, increasing the danger of new cycles of konzo to spread to several other countries in the tropics.
Our ongoing DRC research project seeks methods to prevent and elucidate biomarkers of neurocognition and motor deficits associated with chronic dietary reliance on cyanogenic cassava, a staple food crop for more than 600 million of people living in the tropics. We are implementing a novel cassava processing method that safely removes cyanogenic compounds from cassava flour prior to human consumption. We also develop field-based and affordable diagnostic tools while enhancing the research manpower of the konzo-affected DRC (Democratic Republic of Congo). Field-to-Bench and vice-versa research include research on disease susceptibility focusing on the potential role of the gut functional microbiome and/or epigenetic changes. The overall goal of the cassava-related work meshes well with the global health mission of the NIH while integrating the institute-specific missions of FIC, NIEHS, NICHD, NHGRI, NINDS, NIMH, and the Office of Disease Prevention; the NASA (food crops safety and security agendas and climate change programs), and the UN Sustainable and Developmental goals.
Relevant Publications
- Kakooza-Mwesige, A., Tshala-Katumbay, D., & Juliano, S. L. (Accepted/In press). Viral infections of the central nervous system in Africa. Brain Research Bulletin. https://doi.org/10.1016/j.brainresbull.2018.12.019
- Burfeind, K. G., Kashama, J. M. K., Bora, B. K., Murchison, C. F., Ramos-Crawford, A. L., Nseka, M. T., ... Tshala-Katumbay, D.(Accepted/In press). Baseline characterization of epilepsy in an onchocerciasis endemic area of the Democratic Republic of Congo. Brain Research Bulletin. https://doi.org/10.1016/j.brainresbull.2018.11.009
- Tshala-Katumbay, D., Mwanza, J. C., Rohlman, D., Maestre, G., & Oria, R. B. (2015). A global perspective on the influence of environmental exposures on the nervous system. Nature, 527(7578), S187-S192. https://doi.org/10.1038/nature16034
OHSU Global Southeast Asia
Global Child Health Research Focus

Justin Denny, M.D., M.P.H, is an Associate Professor in the School of Public Health at Oregon Health & Science University (OHSU). There he directs OHSU’s global platform in Southeast Asia, which is based out of Bangkok, Thailand. Together with local partners, Bangkok Dusit Medical Services and Mahidol University, Dr. Denny is responsible for overseeing numerous bodies of work, including the establishment of a center of excellence in occupational health in Southeast Asia; research collaborations looking into new approaches to and understandings for emerging infections; the establishment of a clinical nutrition training program in Laos; an eye project in northern Myanmar to train Burmese residents in sub-speciality eye surgery; and the development of a center of excellence in pediatric care for Southeast Asia. Dr. Denny coordinates faculty and student experiences in each of these areas to ensure a robust, safe and even exchange across OHSU from its schools of Medicine, Nursing, Public Health, Pharmacy and Dentistry.
Prior to leading this effort in Southeast Asia, Dr. Denny worked for five years with the World Health Organization in Laos as deputy team leader of the communicable disease surveillance and response unit. Here his primary role was to create and run the country’s first field epidemiology training program (FET). Through this effort he trained Lao physicians on key concepts in field epidemiology using a one-year training program which emphasized outbreak surveillance and response. As a result, Dr. Denny was able to provide technical and field support to the country for clusters and outbreaks of pandemic and avian influenza, dengue fever, typhoid fever, meningitis, encephalitis, anthrax, malaria, dysentery and acute flaccid paralysis. Many of these efforts led to the creation of national action plans as well as altered vaccination strategies for the country. After the success of the effort in Laos, Dr. Denny was asked to share lessons learned and to work frequently with developing FET programs in Cambodia, Malaysia, Vietnam, Myanmar, Thailand, Vietnam, and Mongolia.
Prior to his time in Southeast Asia, Dr. Denny worked in Sweden as a medical epidemiologist. There he was tasked with coordinating the response to food and water borne communicable disease outbreaks of international interest in the European Union (EU) with the European Center for Disease Control (ECDC). In addition, he was responsible for all human content to the annual EU and European Food and Safety Agency (EFSA) Zoonoses Report and food and water-borne disease content to the ECDC annual report. Dr. Denny produced regular reports on EU food and water-borne disease trends for all EU partners throughout the year, and coordinated yearly meetings for epidemiologists and microbiologists from around the world to share research and outbreak investigation findings.
Relevant Publications
- Xeuatvongsa, A.,Mirza, S., Winter, C., Feldon, K., Vongphrachanh, P., Phonekeo, D., Denny, J, et al. The Lao Experience in Deploying Influenza A(H1N1) Vaccine: Lessons Made Relevant in Preparing for Present Day Pandemic Threats”. PLoS One, 2015 - 10(4), e0121717.
- Hübschen, J. M., Vilivong, K., Souvannaso, C., Black, A. P., Lütteke, N., Samountry, B.,Phonsavath, V., Khamphaphongphane, B., Denny J.,… Muller, C. P. (2014). “High Prevalence of Mumps in Lao People’s Democratic Republic.” Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases, February. doi:10.1111/1469-0691.12586.
- A Dubot-Pérès, P Vongphrachanh, J Denny... An Epidemic of Dengue-1 in a Remote Village in Rural Laos. PLoS neglected tropical diseases. 2013 - dx.plos.org
- Vandy S, Leakhann S, Phalmony H, Denny J, Roces M. Vibrio parahaemolyticus enteritis outbreakfollowing a wedding banquet in a rural village – Kampong Speu, Cambodia, April 2012. WHO Western Pacific Surveillance and Response Journal, 2012, 3(4).doi: 10.5365/wpsar.2012.3.4.004
- J Denny and J McLauchlin. Human Listeria monocytogenes infections in Europe - an opportunity for improved European surveillance. Euro Surveill 2008;13(13)
- J Denny, G Hernandez-Pezzi, J Threlfall, T Westrell, I Fisher. A quarterly update on food- and waterborne diseases in Europe - summary of data for the third quarter of 2007. Euro Surveill 2008;13
- J Denny, J Threlfall, J Takkinen, S Löfdahl, T Westrell, C Varela, B Adak, N Boxall, S Ethelberg, M Torpdahl, M Straetemans, W van Pelt (2007). “Multinational Salmonella Paratyphi B variant Java (Salmonella Java) outbreak, August – December 2007.” Euro Surveill 2007;12(12):E071220.2.
- J Denny, F Boelaert, B Borck, OE Heuer, A Ammon, P Makela (2007). “Zoonotic infections in Europe: trends and figures - a summary of the EFSA-ECDC annual report.” Euro Surveill 2007; 12(12): E071220.6.
- The Community Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents, Antimicrobial Resistance and Foodborne Outbreaks in the European Union in 2006, The EFSA Journal (2007) 130 (Responsible for all human content of publication).
Global Child Health Research Focus

Ms. Leah Cronn is an attorney and is an Assistant Professor and Associate Director with OHSU Global - Southeast Asia. Ms. Cronn co-developed a global program based in Southeast Asia for Oregon Health and Science University (OHSU) which serves graduate students and faculty from the schools of Medicine, Nursing, Public Health, Pharmacy and Dentistry. Currently Ms. Cronn manages over 20 projects for OHSU in SE Asia, funding such projects through public-private partnerships that she developed. Ms. Cronn co-teaches a graduate class of MPH/Ph.d students in public health with a focus on the role of public-private partnerships in global health and global health law. Before joining OHSU, Ms. Cronn served as a deputy district attorney, litigator, corporate counsel, launched a non-profit organization, Marathon Scholars, served on the Board of Directors for The Schlesinger Family Foundation and taught Access to Education at Portland State University.
Public Health
Global Child Health Research Focus

Seth O’Neal is an Assistant Professor of epidemiology in the School of Public Health. He also holds a visiting faculty position at Universidad Peruana Cayetano Heredia in Lima, Peru, and is co-director of the university’s Center for Global Health in Tumbes, Peru.
O’Neal is engaged in research to develop cost-effective and sustainable control interventions for neglected tropical infections in resource-limited settings. His primary focus is on Taenia solium, the pork tapeworm, which is an important cause of preventable epilepsy across much of Latin America, Asia and sub-Saharan Africa. The parasite also perpetuates poverty in these regions by inflicting financial losses on small landowners due to contaminated pork. Through National Institutes of Health and foundation funding, O’Neal conducts community trials of control interventions, as well as clinical and epidemiological studies. His research explores the biological, environmental, social and cultural factors that drive transmission of the parasite, as these affect control interventions.
Relevant Publications
- Fernandez, L., Gamboa, R., Vilchez, P., Pray, I., Beam, M., Garvey, B., ... O'Neal, S. (2019). Evaluating urban taeniasis as a threat to cysticercosis elimination in northern Peru. American Journal of Tropical Medicine and Hygiene, 100(1), 140-142. https://doi.org/10.4269/ajtmh.18-0767
- Garvey, B. T., Moyano, L. M., Ayvar, V., Rodriguez, S., Gilman, R. H., Gonzalez, A. E., ... O'Neal, S. (2018). Neurocysticercosis among people living near pigs heavily infected with cysticercosis in rural endemic Peru. American Journal of Tropical Medicine and Hygiene, 98(2), 558-564. https://doi.org/10.4269/ajtmh.17-0443
- Pray, I. W., Ayvar, V., Gamboa, R., Muro, C., Moyano, L. M., Benavides, V., ... O'Neal, S. (2017). Spatial relationship between Taenia solium tapeworm carriers and necropsy cyst burden in pigs. PLoS Neglected Tropical Diseases, 11(4), [e0005536]. https://doi.org/10.1371/journal.pntd.0005536
Global Child Health Research Focus

Dr. Winthrop’s global health research focuses on elimination and prevention of infectious diseases such as onchocerciasis and tuberculosis (TB). Onchocerciasis, more commonly known as river blindness, is transmitted through bites of black flies and affects children and adults alike in Africa and Latin America. This infectious disease causes devastating outcomes – ocular lesions and ultimately blindness – in untreated individuals. Through his collaboration with the Onchocerciasis Elimination Program for the Americas (OEPA), Dr. Winthrop led epidemiological and clinical training of Latin American ophthalmologists to more effectively target and track onchocerciasis elimination efforts. His efforts were key as part of a large elimination program that has successfully led to the elimination of Onchocerciasis from much of the Americas.
A former infectious disease epidemiologist in the Division of Tuberculosis Elimination at the U.S. Centers for Disease Control and Prevention (CDC) and current medical consultant to the Oregon Public Health Division’s TB control program, Dr. Winthrop has co-authored over 215 publications, many detailing the epidemiologic and clinical aspects of Nontuberculous Mycobacterial diseases (NTM), Tuberculosis (TB), and other infections associated with inflammatory diseases and biologic immunosuppressive therapies used to treat inflammatory conditions. Past clinical research includes a comparison study evaluating the sensitivity and specificity of Purified Protein Derivative (PPD) skin tests for detection of TB, studies involving interferon-gamma release assays including most recently the pivotal phase 3 sensitivity and specificity studies for the newly approved Quantiferon-TB Gold Plus, and numerous epidemiologic studies evaluating the risk of TB in the setting of biologic immunosuppressive therapy. His group has also undertaken novel vaccine research evaluating the effectiveness and safety of zoster vaccination, pneumococcal and influenza vaccinations in the setting of inflammatory disease, and currently a live Dengue vaccine. These studies have helped change clinical practice with regards to the prevention of infection, particularly in the setting of immune mediated inflammatory diseases.
Relevant Publications
- Park JK, Lee YJ, Shin K, Ha YJ, Lee EY, Song YW, Choi Y, Winthrop KL, Lee EB. Impact of temporary methotrexate discontinuation for 2 weeks on immunogenicity of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2018 Jun;77(6):898-904.
- Winthrop KL, Korman N, Abramovits W, Rottinghaus ST, Tan H, Gardner A, Mukwaya G, Kaur M, Valdez H. T-cell-mediated immune response to pneumococcal conjugate vaccine (PCV-13) and tetanus toxoid vaccine in patients with moderate-to-severe psoriasis during tofacitinib treatment. J Am Acad Dermatol. 2018 Jun;78(6):1149-1155.
- Winthrop KL, Wouters AG, Choy EH, Soma K, Hodge JA, Nduaka CI, Biswas P, Needle E, Passador S, Mojcik CF, Rigby WF. The Safety and Immunogenicity of Live Zoster Vaccination in Patients With Rheumatoid Arthritis Before Starting Tofacitinib: A Randomized Phase II Trial. Arthritis Rheumatol. 2017 Oct;69(10):1969-1977.
Vaccine and Gene Therapy
Global Child Health Research Focus

Some T cells are the immune system's memory chips. Before the immune system can mount an effective attack on a disease-causing microorganism, they must form a subset of "memory" T cells specific for that organism. These "memory" T cells orchestrate immune elimination of the invader and remain in the blood and tissues so that the system can mount another counterattack should the foreign agent invade again. Memory T cell activity is also the key to explaining how vaccines work. Vaccines expose the immune system to weakened or noninfectious forms of pathogens, thus activating T cell memory and tricking the system into generating these critical disease-fighting cells. Researchers in Louis Picker's laboratory focus their studies on memory T cell biology in human and nonhuman primates. They investigate the physiology of T cell memory and effector responses, including mechanisms that control "selection" of the memory repertoire, the functional specialization of the memory population, and the elements involved in T cell homeostasis and regeneration. At the same time, these scientists are seeking to determine the basis of effective immunity to certain chronic human pathogens, particularly HIV/SIV, Mycobacterium tuberculosis, and cytomegalovirus, and are working on prophylactic and/or therapeutic vaccines against these pathogens. Dr. Picker and his colleagues have developed special expertise in the quantification and functional characterization of antigen-specific memory T cells. They exploit these technologies in the examination of human subjects and rhesus macaque models of chronic viral infection.
Dr. Picker graduated from UCLA with a B.S. in bacteriology in 1978 and took his M.D. degree at the University of California at San Francisco in 1982. After residency training in pathology at Beth Israel Hospital, Boston, and postdoctoral training in immunology at Stanford University Medical Center, he was appointed assistant professor and then associate professor of pathology at the University of Texas Southwestern Medical Center at Dallas. In 1999 he came to OHSU and ONPRC as professor of pathology/molecular microbiology and immunology in the OHSU School of Medicine and head of the Division of Pathobiology and Immunology.
Relevant Publications
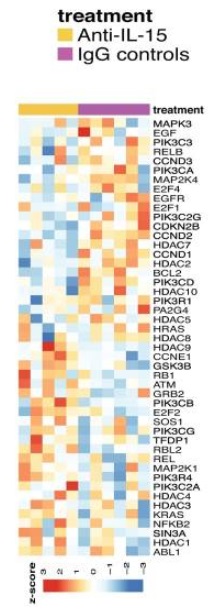
- DeGottardi MQ, Okoye AA, Vaidya M, Talla A, Konfe AL, Reyes MD, Clock JA, Duell DM, Planner SS, Legassse AW, Sabnis A, Park BS, Axthelm MK, Estes, JD, Sekaly R-P, and Picker LJ. Effect of anti-IL-15 administration on T cell and NK cell homeostasis in rhesus macaques, J. Immunol, 197(4):1183-98, 2016. Pre-published online July 18, 2016, doi:10.4049/Jimmunol. 1600065.
- Hansen SG, Zak DE, XuG, Ford JC, Marshall EE, Malouli D, Gilbride RM, Hughes CM, Ventura AB, Ainslie E, Randall KT, Selseth AN, Rundstrom P, HerlacheL, LewisMS, Park H, Planer SL, TurnerJM, Fischer M, Armstrong C, Zweig RC, SylwesterAW, Legasse AW, Messerle M, Jarvis MA, Amon LM, Aderem A, Alter G, Laddy DJ, Stone M, Bonavia A, EvansTG, Messerle M, JarvisMA, Früh K, Axthelm MK, Edlefsen PT and Picker LJ. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine, Nature Med, 24:130-143, 2018; doi:10.1038/nm.4473 (2018).
- Okoye AA, Hansen SG,Vaidya M, Fukazawa Y, Park HM,Duell DM,Lum R, Hughes CM, Ventura AB, Ainslie E, Ford JC, Morrow D, Gilbride RM, Legasse AW, Hesselgesser J, Geleziunas, Li Y, Oswald K, Shoemaker R, Fast R, Bosche WJ, Borate BR, Edlefsen PT, Axthelm MK, Picker LJ*, and Lifson JD*. Early antiretroviral therapy limits SIV reservoir establishment to delay or prevent post-treatment viral rebound, Nature Med, 2018 Aug 6. doi: 10.1038/s41591-018-0130-7. [Epub ahead of print] (*co-corresponding authors)
Global Child Health Research Focus

The Wilder lab is focused on eliminating one of the oldest and deadliest diseases in human history—malaria. Even today, the Plasmodium parasite which causes malaria infects roughly 214 million persons each year and ends the life 400,000 of those. Most malaria deaths occur in children under the age of 5, resulting in approximately 4 times the number of childhood deaths as cancer each year.
The Plasmodium parasite is a eukaryotic organism with a genome many times more complex than most viral or bacterial pathogens. It has been evolving with humans for millennia and as a result has many tricks to evade our immune system. Combined with its complex life cycle which moves between mosquitos, our liver, our blood and back to the mosquitos; combating and even studying malaria is an enormous undertaking.
The Wilder lab is focused on utilizing the unique advantages of OHSU, the Vaccine and Gene Therapy Institute and the Oregon National Primate Research Center to better understand the immunobiology of the Plasmodium parasite and using this to guide novel vaccines and interventions capable of preventing infection. We do this through the use of rodent Plasmodium species in mice, human Plasmodium species in humanized liver mice as well as their closely related non-human primate Plasmodium species in rhesus macaques. These all require use of our newly-constructed ABSL2/ACL2 mosquito insectary which provides the parasites necessary to perform these in vivo as well as in vitro studies.
With these tools, we are primarily focused on identifying new infection-blocking or infection-reducing monoclonal antibodies as well as the mechanisms of their action. This, combined with studying the biology of parasite infection and the resulting immune response can guide us to knowing the parasite vulnerabilities on which to focus the next generation of malaria vaccines—all with the hope that this can bring us closer to finally eliminating this ancient and deadly disease.
Relevant Publications
- A public antibody lineage that potently inhibits malaria infection through dual binding to the circumsporozoite protein. / Tan, Joshua; Wilder, Brandon; Oyen, David; Zenklusen, Isabelle; Piccoli, Luca; Barbieri, Sonia; Foglierini, Mathilde; Fregni, Chiara Silacci; Marcandalli, Jessica; Jongo, Said; Abdulla, Salim; Perez, Laurent; Corradin, Giampietro; Varani, Luca; Sallusto, Federica; Sim, Betty Kim Lee; Hoffman, Stephen L.; Kappe, Stefan H.I.; Daubenberger, Claudia; Wilson, Ian A.; Lanzavecchia, Antonio. In: Nature medicine, Vol. 24, No. 4, 01.05.2018, p. 401-407.
- Immunization of Malaria-Preexposed Volunteers with PfSPZ Vaccine Elicits Long-Lived IgM Invasion-Inhibitory and Complement-Fixing Antibodies. / Zenklusen, Isabelle; Jongo, Said; Abdulla, Salim; Ramadhani, Kamaka; Lee Sim, B. Kim; Cardamone, Hayley; Flannery, Erika L.; Nguyen, Thao; Fishbaugher, Matthew; Steel, Ryan W.J.; Betz, Will; Carmago, Nelly; Mikolajczak, Sebastian; Kappe, Stefan H.I.; Hoffman, Stephen L.; Wilder, Brandon; Daubenberger, Claudia. In: Journal of Infectious Diseases, Vol. 217, No. 10, 23.04.2018, p. 1569-1578.
- Humoral protection against mosquito bite-transmitted Plasmodium falciparum infection in humanized mice. / Wilder, Brandon; Mikolajczak, Sebastian A.; Fishbaugher, Matthew; Vaughan, Ashley M.; Flannery, Erika L.; Nguyen, Thao; Betz, Will; Jane Navarro, Mary; Foquet, Lander; Steel, Ryan W.J.; Billman, Zachary P.; Murphy, Sean C.; Hoffman, Stephen L.; Chakravarty, Sumana; Sim, B. Kim Lee; Behet, Marije; Reuling, Isaie J.; Walk, Jona; Scholzen, Anja; Sauerwein, Robert W.; Ishizuka, Andrew S.; Flynn, Barbara; Seder, Robert A.; Kappe, Stefan H.I. In: npj Vaccines, Vol. 2, No. 1, 27, 01.12.2017.
Uncontrolled inflammation generates significant morbidity and mortality in many non-infectious (e.g. inflammatory bowel disease) and infectious (e.g. HIV) diseases. The central focus of Dr. Estes's research seeks to understand how immune response dysregulation leads to tissue pathology, disease progression, and in the context of infectious diseases, pathogen persistence with the goal to develop and test effective therapeutic strategies that prevent disease and restores immune function. Towards that end, we utilize non-human primate models of several highly relevant human diseases, with a strong emphasis on models of HIV infection and disease, to elucidate determinants of local and systemic inflammation and to test therapeutics that modulate the immune landscape, restore immune homeostasis, and reduce viral reservoirs. We capitalize on the full power of our pre-clinical models by performing cutting-edge in vivo and in situ tissue analyses, including our pioneering work with next-generation in situ hybridization and immunohistochemistry, to generate comprehensive characterizations of the host-pathogen interactions, including the cellular and inflammatory immune landscapes present within relevant tissue microenvironments. Our studies invite collaboration, and we work with academic, government and industry partners around the globe on a wide range of projects from intestinal dysbiosis to the distribution of Zika virus within the host.

Dr. Estes graduated from Brigham Young University with a B.S. in Microbiology and Molecular Biology in 1999. He continued his graduate education at Brigham Young University and obtained his Ph.D. in Immunology and HIV Pathogenesis in 2003 in the laboratory of Dr. Gregory Burton. From 2003 to 2007, he was a postdoctoral fellow in the laboratory of Dr. Ashley Haase in the Department of Microbiology at the University of Minnesota focusing on the in vivo immunopathology of lentiviral infections. Following postdoctoral training, Dr. Estes joined the AIDS and Cancer Virus Program at the Frederick National Laboratory for Cancer Research, and was a Principal Investigator of the Retroviral Immunopathology Section and Senior Principal Scientist and Head of the Tissue Analysis Core. Dr. Estes was appointed an Adjunct Professor in the School of Health and Biomedical Sciences in the College of Science, Engineering and Health at RMIT University in Melbourne, Australia in 2017. In 2017, Dr. Estes accepted a dual appointment as Professor within the Vaccine and Gene Therapy Institute (VGTI) and Chief of the Division of Pathobiology &Immunology in the Oregon National Primate Research Center (ONPRC) at OHSU.
Relevant Publications
Wade, K. S., Estes, J. M., & Kline, R. C. (2020). Genetics and the Gynecologic Patient. Ochsner journal, 20(4), 446–451. https://doi.org/10.31486/toj.20.0051
Barrington, D. A., Champion, M. L., Boitano, T. K. L., Walters-Haygood, C. L., Farmer, M. B., Alvarez, R. D., Estes, J. M., & Leath, C. A., 3rd (2018). Characteristics of African American women at high-risk for ovarian cancer in the southeast: Results from a Gynecologic Cancer Risk Assessment Clinic. Gynecologic oncology, 149(2), 337–340. https://doi.org/10.1016/j.ygyno.2018.02.014
Arend, R. C., Londoño, A. I., Montgomery, A. M., Smith, H. J., Dobbin, Z. C., Katre, A. A., Martinez, A., Yang, E. S., Alvarez, R. D., Huh, W. K., Bevis, K. S., Straughn, J. M., Jr, Estes, J. M., Novak, L., Crossman, D. K., Cooper, S. J., Landen, C. N., & Leath, C. A., 3rd (2018). Molecular Response to Neoadjuvant Chemotherapy in High-Grade Serous Ovarian Carcinoma. Molecular cancer research : MCR, 16(5), 813–824. https://doi.org/10.1158/1541-7786.MCR-17-0594