Our research projects
The overarching goal of our group is to understand the molecular mechanisms governing cytomegalovirus (CMV) replication, with a specific emphasis on understanding how HMCV establishes, maintains and reactivates from latency. In addition, we have pioneered several humanized mouse models to study different aspect of HCMV pathogenesis.
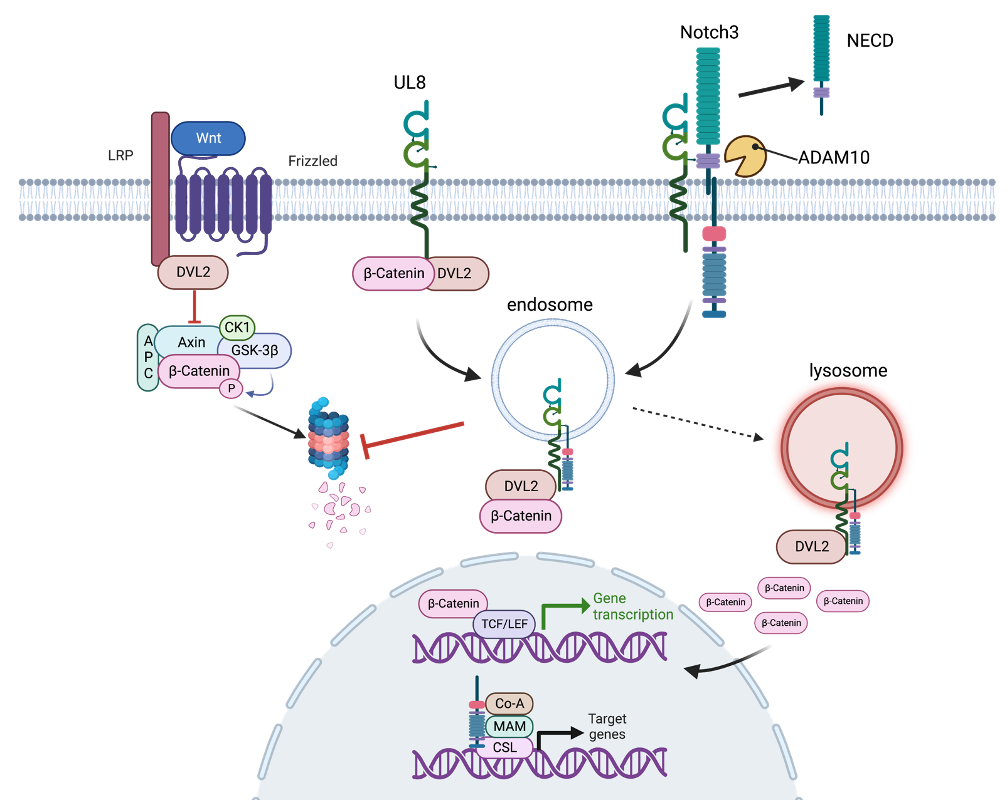
Mechanisms of HCMV pathogenesis. Our group has identified a viral secreted factor, pUL7, that binds the Fms-like tyrosine kinase 3 receptor (Flt-3R), involved in hematopoiesis to promote CD34+ HPC and monocyte differentiation, and ultimately to drive viral reactivation in vitro and in vivo. We recently published that the UL7-related RL11 family member UL8 is also required for efficient viral reactivation in CD34+ HPCs by interacting with components of the (Wnt)/b-catenin pathway. Pull-down experiments demonstrate that UL8 and β-catenin interact with Dishevelled-2 (DVL2) through their PDZ-binding domains, and this interaction promotes b-catenin stabilization and transcriptional activation. Disrupting the interaction between UL8, b-catenin and DVL2 blocks HCMV reactivation in vitro and in vivo, similar to deletion of UL8. Using a proximity-dependent biotinylating enzyme method, we defined the UL8 interactome. We found that UL8 is proximal not only to proteins in the Wnt/b-catenin pathway but also in the Notch pathway. We are currently exploring the role of the Notch pathway in HCMV latency and reactivation, as well as the role played by UL8 in the cross-talk between these two pathways.
Generation of humanized mice for the direct in vivo investigation of viruses with growth restricted to human cells. Our group has developed the first humanized mouse model in which human CD34+ HPC-engrafted NOD-scidIL2Rgc null (huNSG) mice infected with HCMV can support a latent viral infection. Moreover, we have shown that in this model HCMV reactivates in human macrophages following G-CSF-induced mobilization of bone-marrow hematopoietic cells. These data recapitulate observations made in hematopoietic stem cell transplant patients receiving G-CSF mobilized cells from HCMV-seropositive donors. We have extended the HCMV hu-mouse model into NSG mice implanted with bone marrow, liver and thymus (huBLT mice). The huBLT mouse model is superior to conventional humanized mouse models since engraftment of human thymic tissue in these mice supports development of a functional adaptive immune system including functional CD4+ and CD8+ T-cells as well as B-cells with improved class switching. Indeed, we demonstrated that the huBLT model supports HCMV-specific T-cell and antibody responses. Finally, we developed a modified HCMV-huBLT mouse model by engrafting the animals with a minor population of HCMV latently-infected progenitor cells in the context of a larger number of uninfected cells to mimic the common occurance of a small number of CD34+ cells harboring HCMV in seropositive transplant donors. In this model, we demonstrate evidence of HCMV-mediated engraftment failure after hematopoietic cell transplantation, thereby recapitulating the clinical observations and validating the model as a tool for testing novel therapeutics.