Research
Musculoskeletal Tissue Engineering and Regenerative Medicine
Immunomodulatory biomaterials
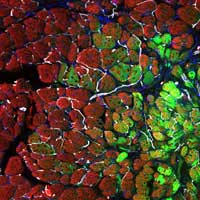
There is an unmet clinical need for off-the-shelf therapeutics to repair and restore function to damaged and diseased tissues. Localized regeneration and long-term recovery depend on a cascade of interactions between the biomaterial and a range of immune cells, stem/progenitor cells, and the tissue microstructure environment. We focus on generating cell-free biomaterials to modulate macrophage polarization, neutrophils, and other key immune players, via biomechanical, chemical, and topographical cues to guide host-driven regeneration and healing.
Rehabilitative exercise

Physical activity is a known influencer of skeletal muscle growth and strengthening. Exercise stimulates blood flow and angiogenesis and proper physical therapy regimens are highly correlative with improved patient outcomes. Our lab combines engineered therapeutics with regenerative rehabilitation to enhance mechanically stimulated reparative pathways in damaged tissues.
Interface engineering
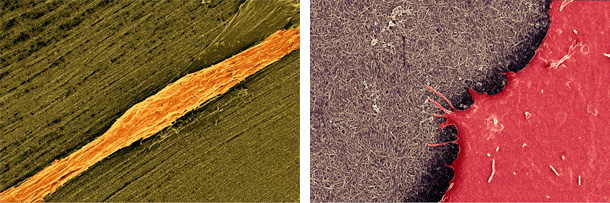
Traumatic musculoskeletal injuries are associated with complex tissue damage particularly at the interface of muscle and bone. Although skeletal muscle exhibits known self-reparative properties, once a critical volume of muscle is lost, the tissue cannot independently restore the injured muscle, resulting in extensive scar formation and functional impairment. Furthermore, traumatic musculoskeletal injuries commonly present with complex blast or impact damage to both bone and soft tissues. A major challenge facing new therapeutics is the development of stratified and gradient biomaterials to bridge these interfaces. Using regional spatial, compositional, and mechanical cues to differentially regulate cell fate and phenotypic maintenance, we have the dual goals of augmenting soft tissue regeneration while also restoring load-bearing tissue architecture to generate cohesive and physiologically mimetic vascularized bone-ligament/tendon-muscle composites.
The Nakayama Laboratory works on developing engineered and regenerative therapies to treat cardiovascular and musculoskeletal injuries and disease. Through modulation of biomaterial properties and cellular interactions, we engineer cells, tissues, and the regenerative response to improve healing and advance quality of life.
Engineering cardiovascular tissues
Vascular grafts
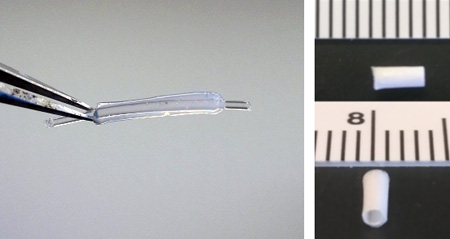
Through biophysical modulation of material scaffolds, we can modulate the phenotype and inflammatory potential of vascular endothelial cells (ECs) for the generation of small diameter vascular grafts that exhibit resistance to the development of atherosclerotic lesions. We have examined extracellular matrix based graft materials (Image 1a) as well as electrospun hybrid polymers (Image 1b) in the context of in vitro iPSC-derived cell guidance and in vivo as interposition carotid artery grafts. We have shown that through spatial patterning of these graft materials, healthy function and anti-inflammatory EC phenotype can be controlled. We are continually exploring new ways to use biomaterials to modulate vascular cell function and improve grafting outcomes.
Physiologically mimetic environment devices

A key challenge facing the translation of engineered tissues into clinical therapeutics may be in part due to conducting our biological questions and building target tissues in physiologic isolation. We are interested in understanding the impact of flow on endothelial cells when complexed with spatial patterning and mechanical gradients in both 2 and 3 dimensions using physiologically mimetic flow devices to better understand the development of atherosclerotic lesions and failure of clinically adopted grafting approaches and ECMO devices. Expounding on the importance of physiological context, we are interested in combinatorial interactions between multiple tissue systems under dynamically regulated biophysical conditions.