Projects
Hypertension
Role of pericyte angiotensin receptor signaling in kidney disease
Pericytes are an important vascular cell type in the kidney. In addition to supporting endothelial cells in capillaries, they also have specialized functions in the glomerulus, where they are referred to as mesangial cells.
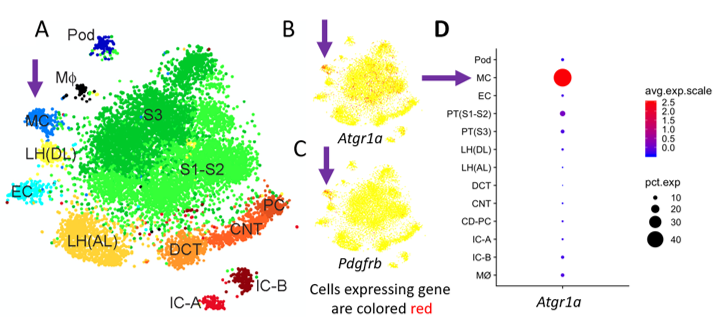
Recent single-cell RNAseq datasets have revealed that pericytes express abundant amounts of the type 1a angiotensin receptor (Figure 1). We hypothesize that this means that pericytes are playing a major role in kidney disease.
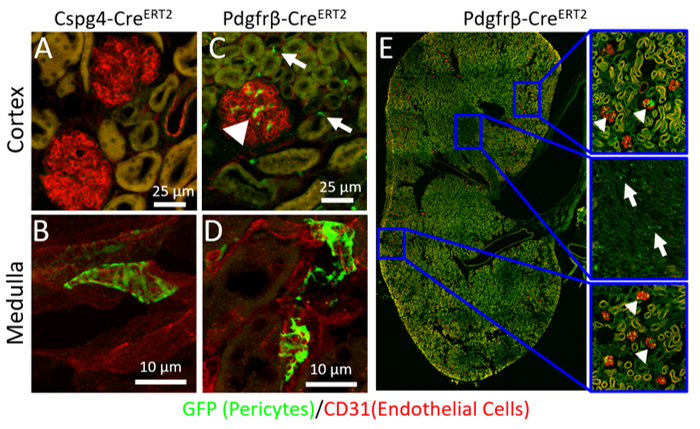
Our lab is exploring the role of pericyte angiotensin receptor signaling in kidney disease by conditionally knocking out the angiotensin receptor from pericytes with the Pdgfrb-creERT2 (Figure 2). We are looking at how this affects kidney phenotypes in both Angiotensin II induced hypertension as well as diabetic kidney disease.
This project is supported by the NIDDK (1K01DK121737) AHA (20CDA35320169)
ACE2
ACE2
ACE2 is highly expressed in the renal proximal tubule (PT) and endothelial cells. What are the critical pools of ACE2 and how does soluble ACE2 contribute to blood pressure regulation?
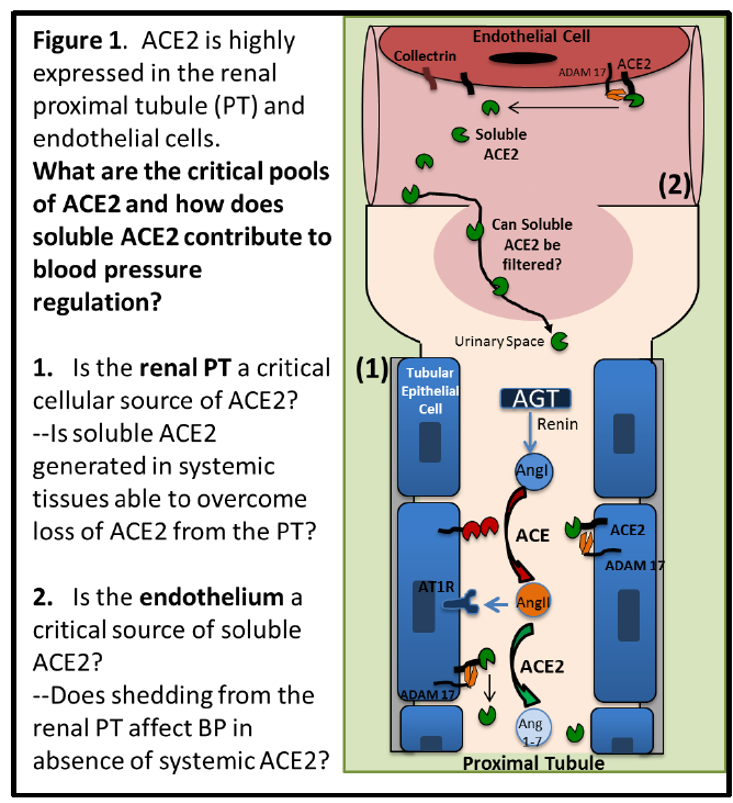
2. Is the endothelium a critical source of ACE2? Does shedding from the renal PT affect BP in absence of systemic ACE2?
Kidney Disease
Pericytes in Diabetic Kidney Disease
Diabetic kidney disease (DKD) is the leading cause of end-stage renal disease (ESRD) in the United States and around the world. In Oregon, it affects thousands of residents making them reliant on renal replacement therapy such as dialysis or kidney transplant. Unfortunately, very few therapies delay the progression of DKD towards ESRD. The major therapeutic strategy to delay progression of DKD is pharmacologic inhibition of the renin angiotensin system (RAS) with angiotensin-converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs). Use of these drugs highlights the importance of RAS signaling in the pathophysiology of DKD and also highlights the need for additional therapies to prevent thousands of Oregonians from a debilitating reliance on dialysis or the uncertainty of waiting for kidney transplant.
Our research group has developed a novel mouse model of DKD, combining type 1 diabetes with chronic, low-grade activation of the RAS, which together recapitulates key features of human DKD including alterations to kidney function and kidney structure. This mouse model is an ideal platform for studying the molecular mechanisms that drive DKD and testing out novel therapeutic strategies for preventing and reversing DKD.
We recently found that a key receptor in the RAS signaling pathway (drug target of ARBs) is abundantly expressed in a specialized cell type of the kidney. Specifically, we found that the type 1 angiotensin receptor (AT1aR) is abundantly expressed within renal mesangial cells. This finding correlates with two well-defined early pathological features of DKD; albuminuria and mesangial expansion. Furthermore, it demonstrates that the cell type with abundant expression of AT1aR is localized to the glomerulus, the anatomical location with characteristic diabetic structural changes to kidney tissue. Taken together, this implicates RAS signaling through mesangial cells as a contributor to the pathophysiology of DKD, however, specific action of AT1aR in mesangial cells has not been examined before.
This project is funded by the Collins Medical Trust.