Projects
Small molecules to overcome treatment resistance
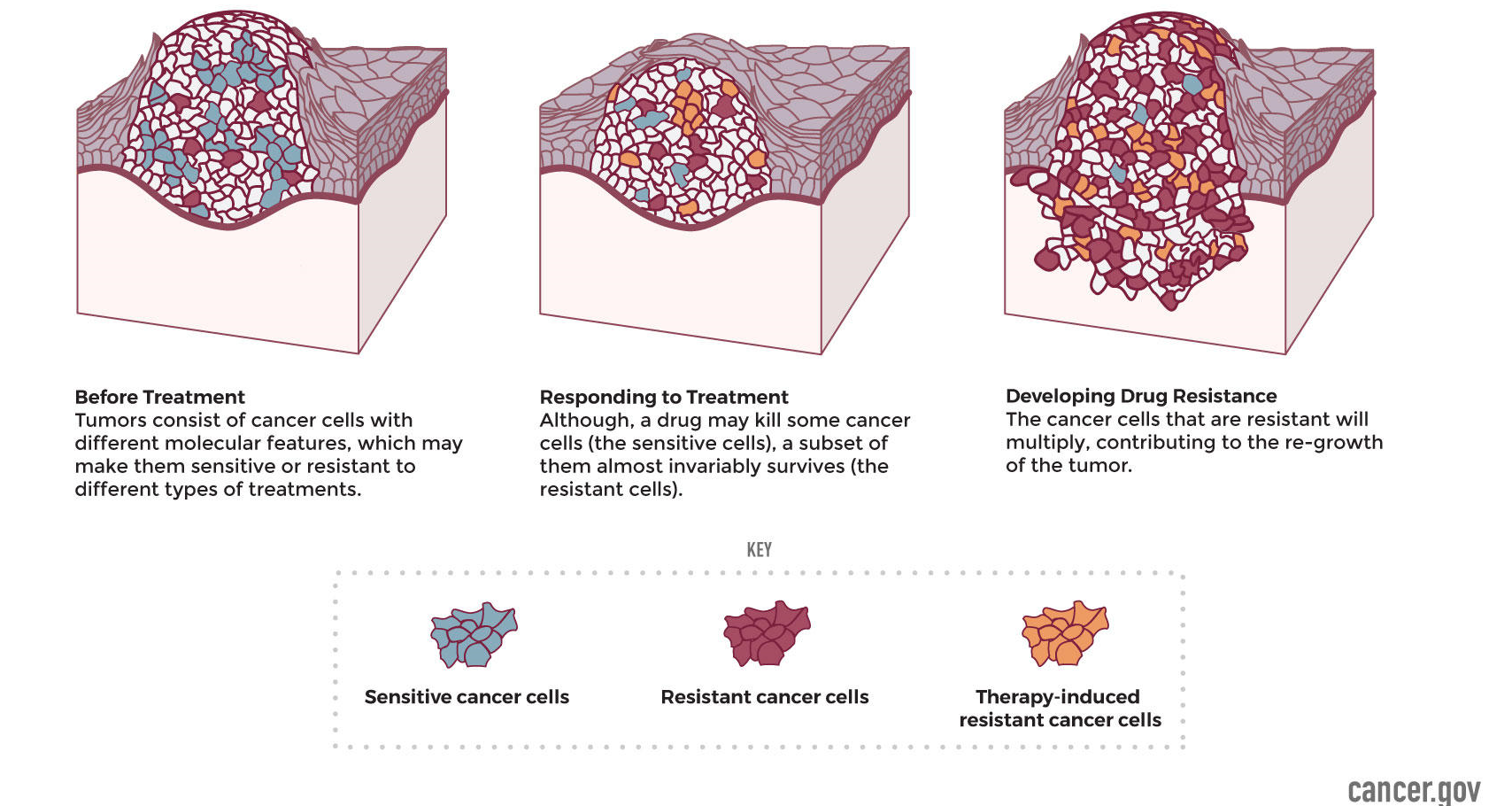
Resistance to chemo-, radio-, or immuno-therapies ultimately underlies all cancer-related mortality. This resistance may be intrinsic before treatment even begins or instead emerge in response to treatment as a selective pressure. Treatment resistance manifests clinically as continued disease progression even with ongoing treatment. Investigation of patient-derived samples and models has identified many resistance-associated targets whose characterization has revealed new insights into cancer progression and treatment efficacy. New targets and associations between them are being revealed by ongoing efforts and large datasets. Combining cutting-edge medicinal chemistry with molecular cancer biology, the Malhotra lab is investigating treatment resistance in cancer on multiple fronts. First, by working with established resistance-associated targets, we generate and screen new small molecules to develop promising drug candidates and characterize their mechanism of action. Second, by working with known drugs and making modifications, we develop new compounds with optimized bioavailability, toxicity, and pharmacokinetic parameters. Third, by working with clinical collaborators and emerging models, we interrogate resistance-associated biology to identify, validate, and then begin the process of drugging new resistance-associated targets. These efforts combine in an overarching goal of the laboratory: to develop effective new drugs and companion molecular diagnostics to treat and monitor treatment resistance in ovarian, breast, head-and-neck, and other cancers.
Developing novel Nrf2 activators and inhibitors to study their effects on Nrf2 regulation, its activation and therapeutic applic
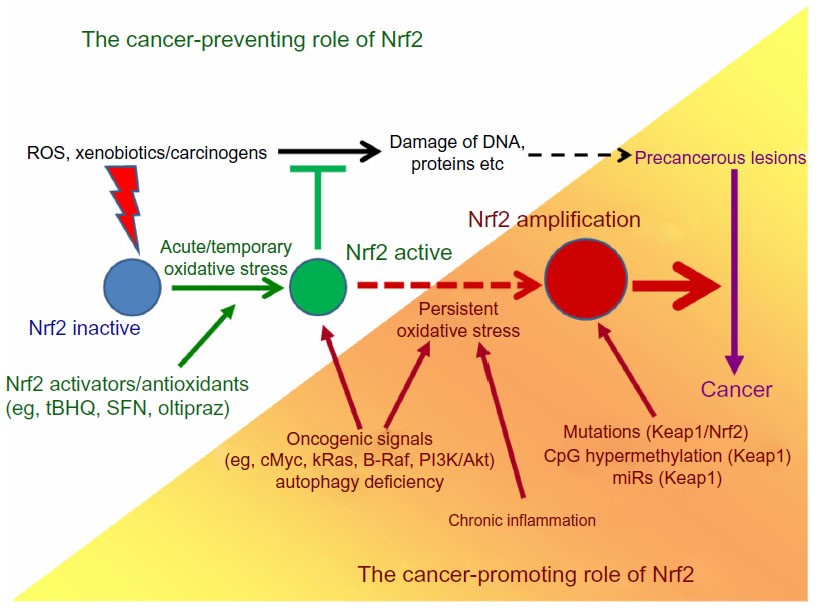
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a redox-sensitive master regulatory transcription factor. It plays an important role in the antioxidant response pathway triggered by oxidative stress and chemotherapeutic drug-induced cytotoxicity. Nrf2 in particular regulates the expression of antioxidant genes, such as heme oxygenase 1 (HO-1), glutamate cysteine ligase catalytic subunit (GCLC), and NAD(P)H dehydrogenase quinone 1 (NQO-1) which neutralize intracellular accumulation of reactive oxygen species (ROS). Under normal conditions, Keap1 retains Nrf2 in the cytoplasm and represses its activity by binding to its Neh2 domain and consequently promoting contact between Nrf2 and the Cul3/Rbx1 ubiquitin ligase complex, leading to ubiquitination of Nrf2 by proteasomal degradation. Under oxidative stress conditions, the Nrf2-Keap1 interaction is disrupted by modification of Keap1 allowing the release of Nrf2 from Keap1 and its translocation from cytoplasm into the nucleus, where Nrf2 binds to the antioxidant response elements (ARE) in the upstream UTR of promoter regions and initiating the transcription of antioxidant response genes. Nrf2-mediated cytoprotective gene induction is a highly effective strategy for reducing susceptibility to carcinogens. It has been shown that Nrf2 knockout mice exhibit increased sensitivity to many diseases including cancer. A wide variety of dietary and synthetic compounds that function as potent inducers of ARE-regulated gene expression, e.g. sulforaphane, dithiolethione and curcumin, have been shown to exert chemopreventive activities. Studies have shown that Nrf2 activators possess radio-protective effects in variety of cells, facilitated by increasing DNA repair response and neutralizing ROS. Based on these studies we discovered novel chalcone-based Nrf2 activators. When administered orally, our lead compound, 2-trifluoromethyl-2’-methoxychalone (TMC), mitigated myelosuppression and mortality in mice even after 24 hours of lethal IR exposure. Our lab is actively working on developing the Nrf2 activators and explore their mechanism; and therapeutic application in radiation-prevention.
Role of Nrf2 in cancer drug resistance
The Nrf2/ARE signaling pathway plays an important role in cancer cell survival and drug resistance. A number of malignant tumors, for example, colonic, thyroid, endometrial, lung, ovarian, breast and pancreatic cancers, exhibit an increased Nrf2 expression and constitutive activation. This enhanced Nrf2 activation originates from rare gain-of-function mutations of Nrf2 itself, and from loss-of-function mutations, epigenetic promoter hypermethylation or increased expression of microRNAs targeting the Nrf2 inhibitory protein Keap1/INRF. As a consequence of the enhanced Nrf2 activity, tumor cells acquire protection from apoptosis and are more capable of proliferation, both conditions favoring tumorigenesis and also making tumor cells more refractory to chemo- and radiotherapy. This suggests that the development of novel Nrf2 inhibitors used in combination with existing anticancer drugs could be a rational strategy to combat chemoresistance. We are developing new small molecule-based Nrf2 inhibitors and studying their application in sensitizing the chemotherapeutic effect of anticancer drugs in triple negative breast cancer (TNBC) model to overcome chemoresistance.
Image credit: OncoTargets and Therapy 2014:7 1497–1518
The development of allosteric modulators of cannabinoid receptor type 1 (CB1) for the treatment of neuropathic pain
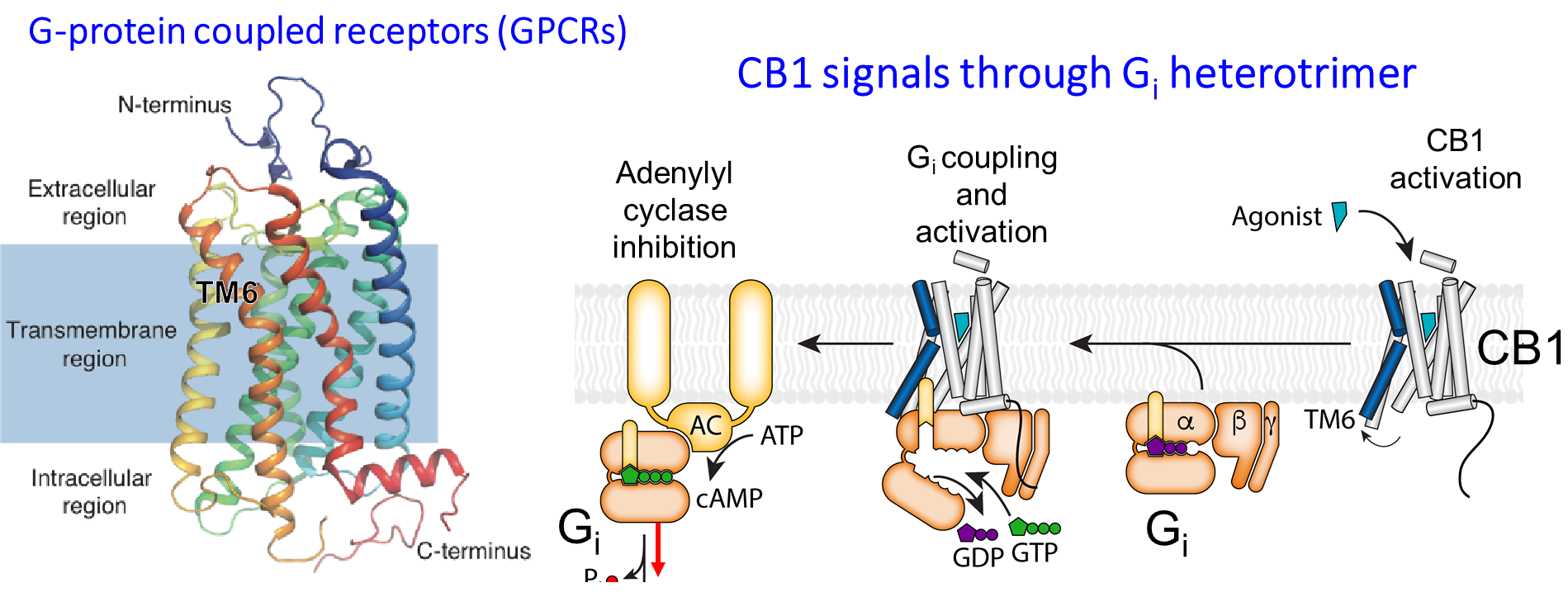
Neuropathic pain is a complex, chronic pain state arising as a direct consequence of a lesion or disease affecting the somatosensory nervous system, and is common to conditions such as back and neck pain, diabetic peripheral neuropathy, and fibromyalgia. Neuropathic pain is estimated to affect 1 in every 10 adults over the age of 30, however, only a third of sufferers experience even partial relief from the currently prescribed treatments, and 30% report dose-limiting side-effects.
Agonists of cannabinoid receptor type 1 (CB1), such as the Δ9-tetrahydrocannabinol (THC) found in cannabis, demonstrate efficacy in preclinical rodent models and in clinical neuropathic pain sufferers. However, the broad therapeutic application of cannabinoid agonists is hindered by dose-limiting intoxication, hypothermia, and tolerance.
Agonist-mediated activation of CB1 at the orthosteric site leads to a diverse array of signaling mechanisms predominantly via G proteins of the Gi/o family (Gi1, 2, and 3, and Go1, and 2), phosphorylation of extracellular signal-regulated kinases (ERK), and β-arrestin recruitment. Although selective activation of CB1 signaling pathways could mitigate adverse effects, most orthosteric agonists induce active CB1 conformations that demonstrate little signaling bias. Very recently, a small molecule targeting a purported allosteric site on CB1 was found to be highly efficacious in a rodent model of neuropathic pain, and without induction of psychoactive or hypothermic effects.
We are designing positive allosteric modulators (PAMs) of CB1 that induce active conformations of the receptor to increase the tone of basal endocannabinoid CB1 signaling in a functionally selective way that minimizes adverse effects. Since the β-arrestin signaling pathway is associated with desensitization, CB1 internalization, and tolerance, we are developing biased PAMs capable of enhancing G protein signaling rather than β-arrestin recruitment. The rational design of functionally selective PAMs will deliver new therapeutic candidates for the treatment of neuropathic pain.
The design of selective cannabinoid receptor type 2 (CB2) ligands for the treatment of glioblastoma
Glioblastoma is the most common—and most aggressive—of all cancers originating in the brain. The current standard of care includes a combination of surgical resection of tumor, radiotherapy, and chemotherapy (temozolide). Even with treatment, the survival rate from diagnosis rarely exceeds 12-15 months. The development of new chemotherapeutic agents with improved efficacy and tolerability represent an important clinical need for glioblastoma patients.
The cannabinoid receptor type 2 (CB2) is upregulated in glioblastoma, and its expression has been shown to correlate with tumor aggressiveness. We have demonstrated that non-psychoactive phytocannabinoids and CB2-selective terpenes are cytotoxic against several glioblastoma cell lines in vitro, and are well tolerated by healthy cells. Using these natural products as leads, a drug discovery program focusing on improved potency and pharmacokinetic properties of CB2-selective small molecules is underway.