News from OHSU Casey Eye Institute
Read more about the latest in patient care, education and research from OHSU Casey Eye Institute.
Connect with us
Outreach

New mobile eye clinic means more vision-saving care
Donations enable OHSU Casey Outreach Program to obtain second mobile unit
Research
Stem Cell Research Offers Hope for Future Glaucoma Treatment
Stem cells could revolutionize both early detection and treatment of glaucoma.
Research
Pioneering Optoretinography
Capturing photoreceptor dysfunction in a relatively wide area of the macula in Patients with Retinal Disease.
Research
Retinal Stem Cell Center offers hope
New Center Brightens the Future for People with Inherited Retinal Diseases.
News

President Biden honors David Huang, M.D., Ph.D.
Huang recognized with National Medal of Technology and Innovation for co-inventing transformative technology
News
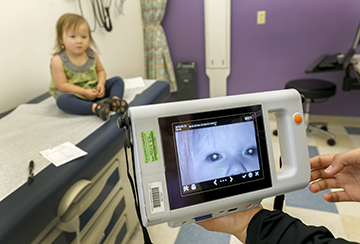
OHSU program that protects kids’ vision marks 20 years
Elks Preschool Vision Screening Program has screened more than 65,000 Oregon children since 2003.
News
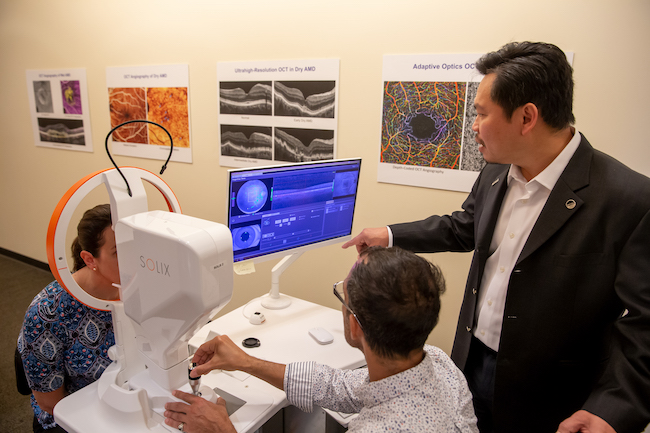
David Huang, M.D., Ph.D., receives Lasker Award
Prize recognizes wide use of optical coherence tomography to manage eye disease, prevent blindness.
Outreach
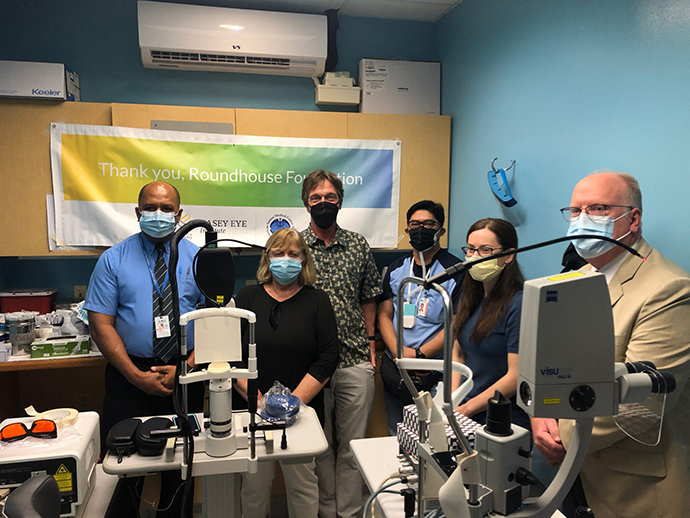
Casey and Local Partners Join Forces in American Samoa
About 2,500 miles from its nearest neighbor, Hawaii, American Samoa is as remote as it gets. That’s...
Outreach
Donors Help Expand Vision Care Statewide
A new way of practicing eye care could have a profound effect on our goal to end preventable blindness in...
News

Gene therapy allows teen to see snowflakes for first time
OHSU Casey Eye Institute part of clinical trial evaluating potential treatment for inherited blindness.
News

Innovations 2023
Highlights of Casey Eye Institute's transformative work in ophthalmology research and clinical care, which...
Outreach
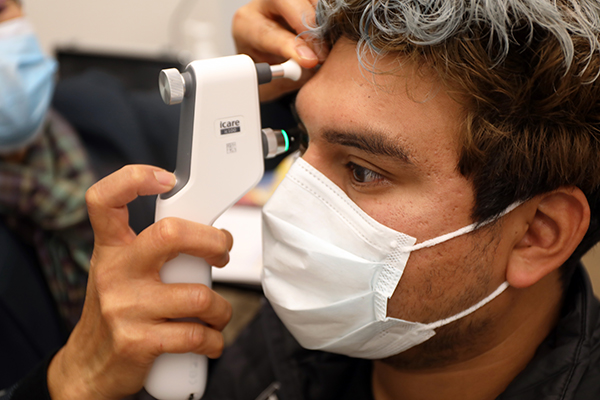
Oregon Vision Health Network expands vision care statewide
The Oregon Vision Health Network is expanding our model of eye care from individual to population health.
Research
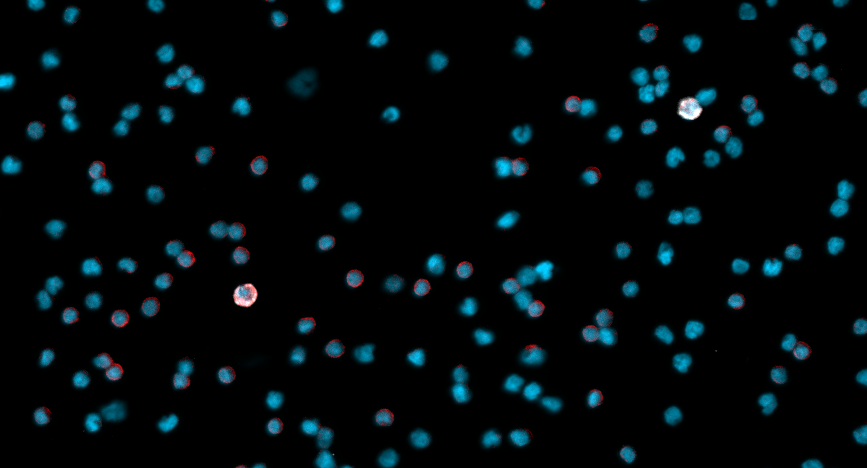
New clues to metastasis of Ocular Melanoma
A collaboration among OHSU cancer researchers reveals which cases of ocular melanoma are likely to spread.
Research
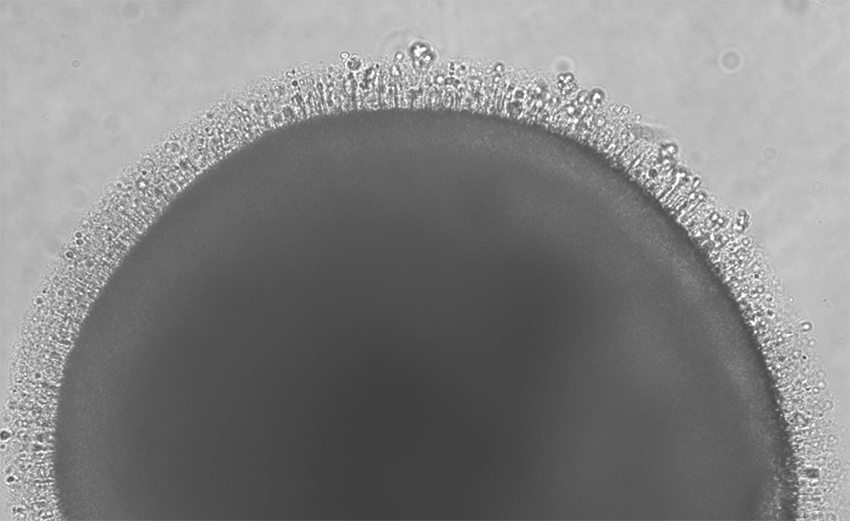
New stem cell center advances retinal disease research
The new center explores exciting possibilities for treating inherited retinal diseases that lead to blindness.
News

Dr. David Huang joins National Academy of Engineering
David Huang, M.D., Ph.D., recognized for co-creating widely used medical imaging technology.
News

OHSU confirms first nonhuman primate model of Usher syndrome
Researchers now have a model for testing gene therapy as a treatment for leading cause of blindness-deafness.
News
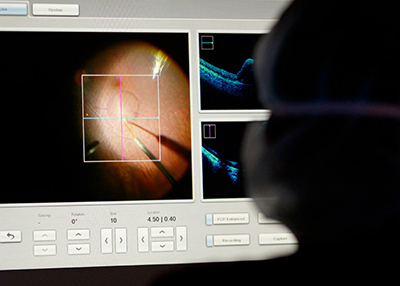
Nanotechnology may improve gene therapy for blindness
New OHSU, OSU research uses lipid nanoparticles to target light-sensitive cells in the eye.
Clinical care

Finding new ways to protect and heal the cornea
The eye is known as the window to the body, and the cornea is known as the window to the eye. The clear...
News

A Grand Opening, At Last
On October 8 the Casey community and the Oregon State Elks Association were finally able to celebrate the...
News

Andreas Lauer named director of the Casey Eye Institute
OHSU School of Medicine Interim Dean David Jacoby has appointed Andreas K. Lauer, M.D., director of the...
Clinical care
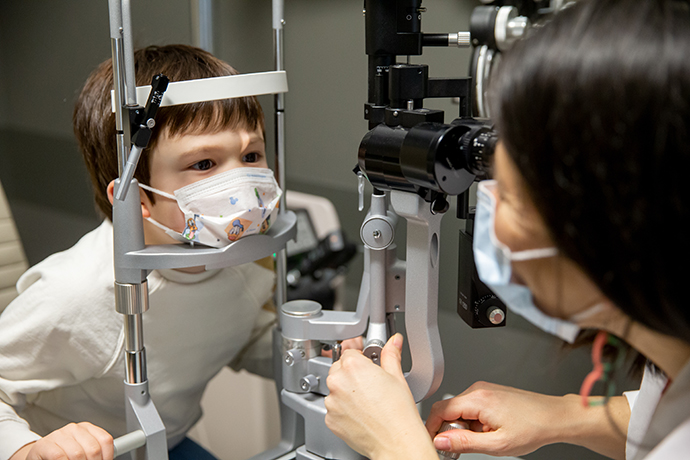
Myopia on the rise, especially among children
Myopia, also known as nearsightedness, is on the rise in the U.S. and around the world—particularly among....
Clinical care

Innovative diagnostic test catches rare eye cancers
The COVID pandemic, has posed particular challenges for research activities at Casey, and all of OHSU...
Clinical care

Expediting care for adults and children with ocular impacts
Facial Nerve Center is the only place in Oregon to offer cutting edge facial reanimation surgery.
Research
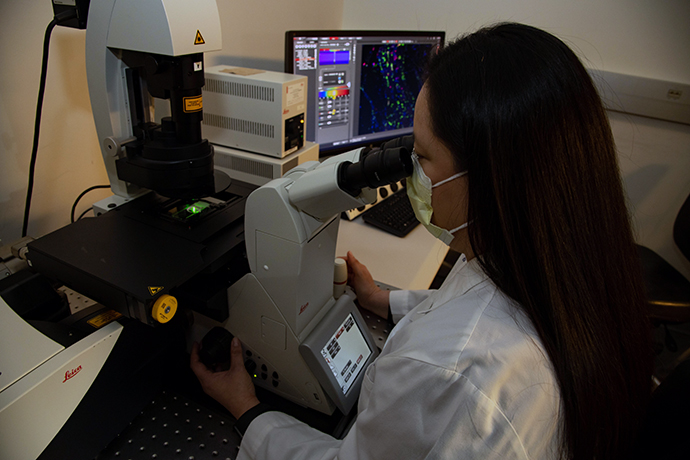
Extraordinary teamwork generates breakthroughs in glaucoma
Glaucoma researchers at OHSU Casey Eye Institute work cooperatively to improve glaucoma care. Many...
Research
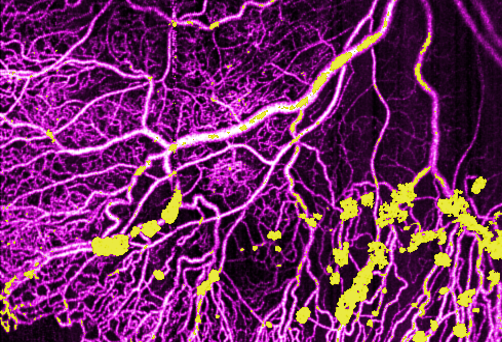
Ultrawide-field research represents next evolution of OCT
OHSU Casey Eye Institute is a leader in noninvasive imaging technology to detect vascular changes in the eye..
Clinical care
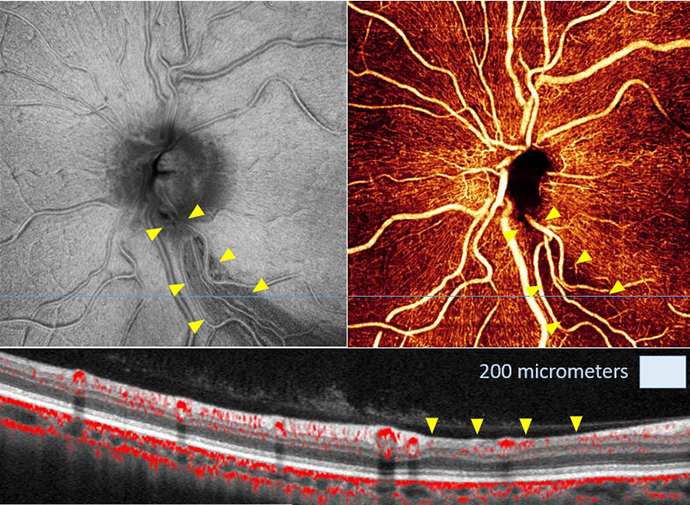
Finding the other 50 percent of patients with glaucoma
How can we improve the evaluation and care of patients with glaucoma? Early diagnosis is key.
Clinical care
A Disco in the Sky
Casey patient JoDee Hambright can see stars for the first time thanks to gene therapy.
Medical education

Residency program expansion strengthens learning and service
Adding a new class of residents improves ophthalmology training experience.
News

OHSU trains community health workers for statewide network
Portland-based OHSU Casey Eye Institute team collaborates with community health clinics across Oregon to...
News
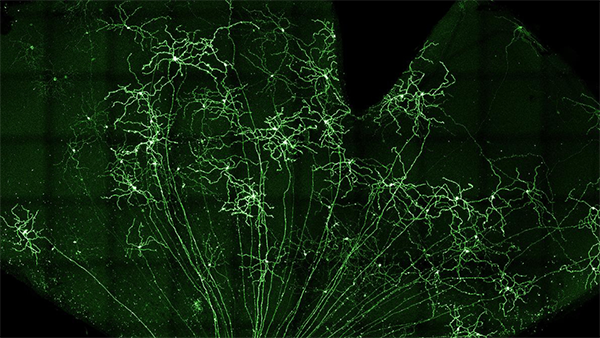
Study investigates stem cell therapy to treat glaucoma
NIH awards $6.7M total to research team that includes OHSU Casey Eye Institute
News

Clinical trial participant dreams of a future with sight
Oregon woman with impaired vision hopes medical advances will mean children with CEP290 gene mutations...
News

Oregon donors help OHSU expand access to eye care statewide
Philanthropic gifts totaling $3.25 million will support OHSU Casey Eye Institute Community Outreach Program.
Research
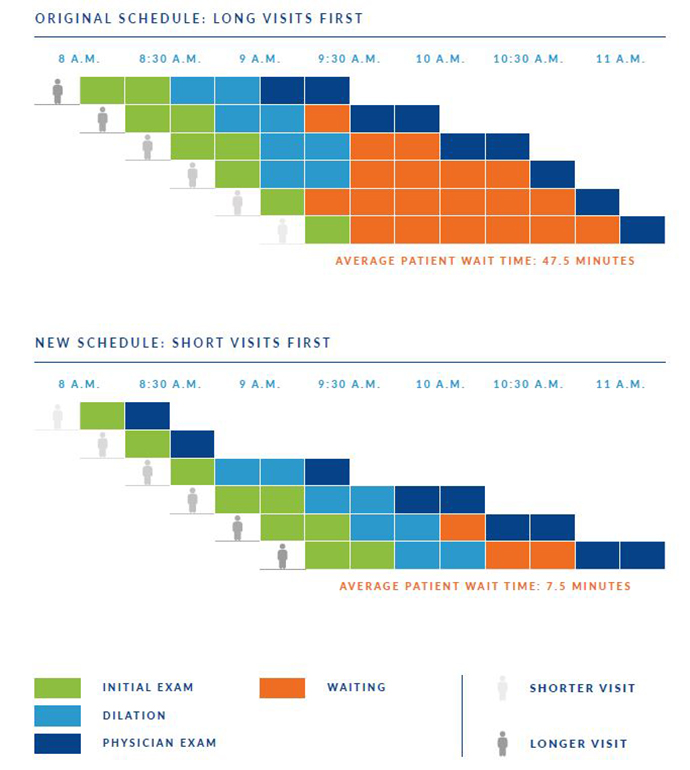
Using data analytics to solve real-world workflow challenges
OHSU Casey Eye Institute is a pioneer in the use of computers to support patient care. We were among the...
Research
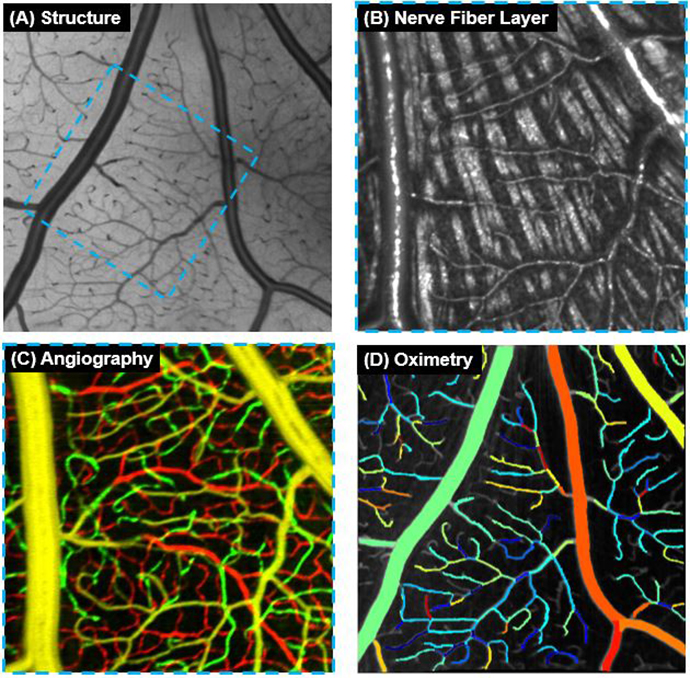
Using spectroscopic OCT to measure capillary oximetry
Ophthalmic imaging expert Yali Jia, Ph.D. and her team constructed a spectroscopic optical coherence...
Research
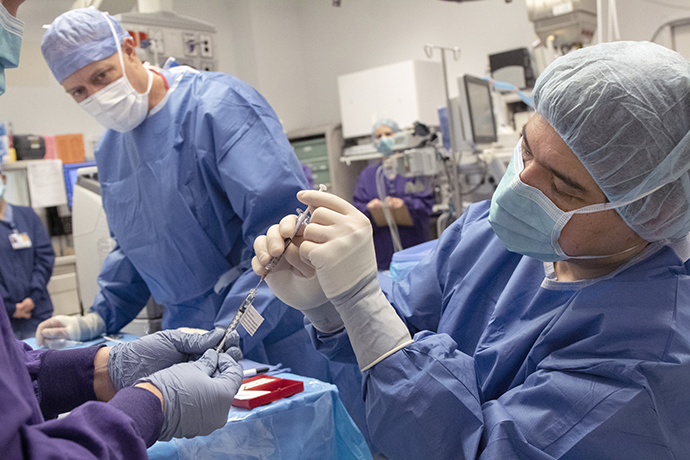
Pioneering the first-ever CRISPR gene editing in vivo
On Feb. 25, 2020, OHSU Casey Eye Institute led a unique moment in human history—the first gene editing...
Research
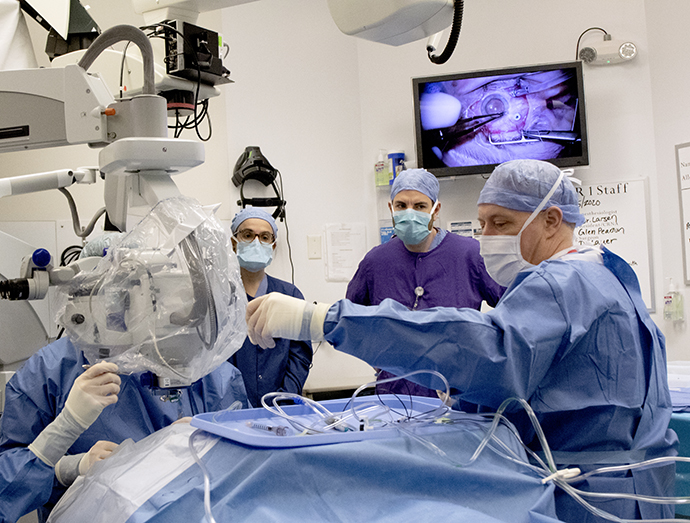
OHSU performs its first stem cell therapy in the retina
The OHSU Casey Eye Institute has used a stem cell therapy in the retina for the first time as part of a...
News

Oregon Elks Children’s Eye Clinic opens its doors
New facility is welcoming space where sight-saving research, patient care and outreach can flourish.
News

OHSU physician honored for co-inventing imaging technology
David Huang, M.D., Ph.D., recognized by Sanford and Susan Greenberg Prize to End Blindness.
News

10,000+ free eye exams to prevent blindness in Oregon
OHSU Casey Community Outreach Program’s mobile eye clinic celebrates 10 years.
News

OHSU ophthalmologist to lead National Eye Institute
Michael Chiang, M.D., will begin new role at NIH research institute in late 2020.
News

OHSU performs first-ever CRISPR gene editing in human body
BRILLIANCE clinical trial aims to enable sight in people born with a blindness-causing mutation.
News

Tech detecting cause of preemie blindness gets federal nod
Artificial intelligence algorithm receives FDA breakthrough device status.
News

FDA recommends approval of eye drug tested in Portland
OHSU is part of multi-center study testing teprotumumab for thyroid eye disease.
News

Discovery in monkeys could lead to treatment for blindness
Oregon National Primate Research Center at OHSU reports first-ever nonhuman primate model for Bardet-Biedl...
News

Lewis & Clark student advances Usher’s syndrome research
Junior biology major works to ‘solve my own problem and also help others’.
News

Occupational therapist helps her peers find independence
OHSU’s Kathryn Marxen-Simonson teaches use of tech, tools to improve quality of life.
News

Dr. David Wilson inaugural recipient of Paul H. Casey Chair
David Wilson, M.D., was appointed the inaugural recipient of the Paul H. Casey Chair in Ocular Oncology...
News

New eye clinic building meets construction milestone
Last beam raised to top out Elks Children’s Eye Clinic.
News

High hopes for 4-year-old’s vision after gene therapy
Portland boy among first in Oregon to have new treatment for rare, blindness-causing genetic mutation.
News

Elks association essential to OHSU's fight against blindness
Fraternal order gives $20M to build new Oregon Elks Children’s Eye Clinic.
News

Telemedicine accurately diagnoses cause of rare blindness
OHSU study find little difference between in-person, remote exams.