Welcome!

The Vollum Institute is a privately endowed research institute at Oregon Health & Science University dedicated to basic research that will lead to new treatments for neurological and psychiatric diseases. Vollum scientists have broad-ranging interests that coalesce around molecular neurobiology and cellular physiology. Their work has transformed the field of neuroscience and, in particular, has provided important advances in the study of synaptic transmission, neuronal development, neurotransmitter transporters, ion channels and the neurobiology of disease.
Learn more about the Vollum's mission
Vollum Seminar Series
Friday Work–In–Progress Talks
The Friday "work-in-progress" (WIP) seminars occur weekly during the academic year and provide an opportunity for postdoctoral fellows and graduate students to share their current research projects in an interactive and less formal environment.
Find out more information on how to attend and the speaker schedule.
Research highlights
Synaptic input and Ca2+ activity in zebrafish oligodendrocyte precursor cells contribute to myelin sheath formation.
Li J, Miramontes TG, Czopka T, Monk KR. (2024) Nat Neurosci. 1038/s41593-023-01553-8.
The Na+ leak channel NALCN controls spontaneous activity and mediates synaptic modulation by α2-adrenergic receptors in auditory neurons.
Ngodup T, Irie T, Elkins SP, Trussell LO. (2024) Elife. 12:RP89520.
Inhibitory CCK+ basket synapse defects in mouse models of dystroglycanopathy.
Jahncke JN, Miller DS, Krush M, Schnell E, Wright KM. (2024) Elife. 12:RP87965.
Astrocyte growth is driven by the Tre1/S1pr1 phospholipid-binding G protein-coupled receptor.
Chen J, Stork T, Kang Y, Nardone KAM, Auer F, Farrell RJ, Jay TR, Heo D, Sheehan A, Paton C, Nagel KI, Schoppik D, Monk KR, Freeman MR. (2024) Neuron. 112(1):93-112.e10.
Functional maturation of the rod bipolar to AII-amacrine cell ribbon synapse in the mouse retina.
Kim MH, Strazza P Jr, Puthussery T, Gross OP, Taylor WR, von Gersdorff H. (2023) Cell Rep. 42(11):113440.
Single-cell RNAseq analysis of spinal locomotor circuitry in larval zebrafish.
Kelly JJ, Wen H, Brehm P. (2023) Elife. 12:RP89338.
Calcium-sensitive subthreshold oscillations and electrical coupling in principal cells of mouse dorsal cochlear nucleus.
Hong 洪卉 H, Moore LA, Apostolides PF, Trussell LO. (2024) J Neurosci. JN-RM-0106-20.
Cryo-EM structures reveal native GABAA receptor assemblies and pharmacology.
Sun C, Zhu H, Clark S, Gouaux E. (2023) Nature. 622(7981):195-201.
Molecular architecture of TMEM63 mechanosensitive ion channels.
Syrenne JT, Sonawane PJ, Murthy SE. (2023) Cell Calcium. 115:102798.
OHSU scientists awarded funding to extend leading-edge research
Recipients of the 2024 Faculty Excellence and Innovation Awards, made possible by the Silver Family Innovation Fund, include Angelica Morales, Ph.D., Arpiar “Arpy” Saunders, Ph.D., and Zheng Xia, Ph.D.
Each recipient receives a total of $750,000 over three years. The innovation fund is designed to buoy the next generation of faculty leaders at OHSU, so awardees are early- or mid-stage investigators of exceptional creativity and promise. This marks the fifth year since the annual awards began in 2020.
Study reveals function of little-understood synapse in the brain
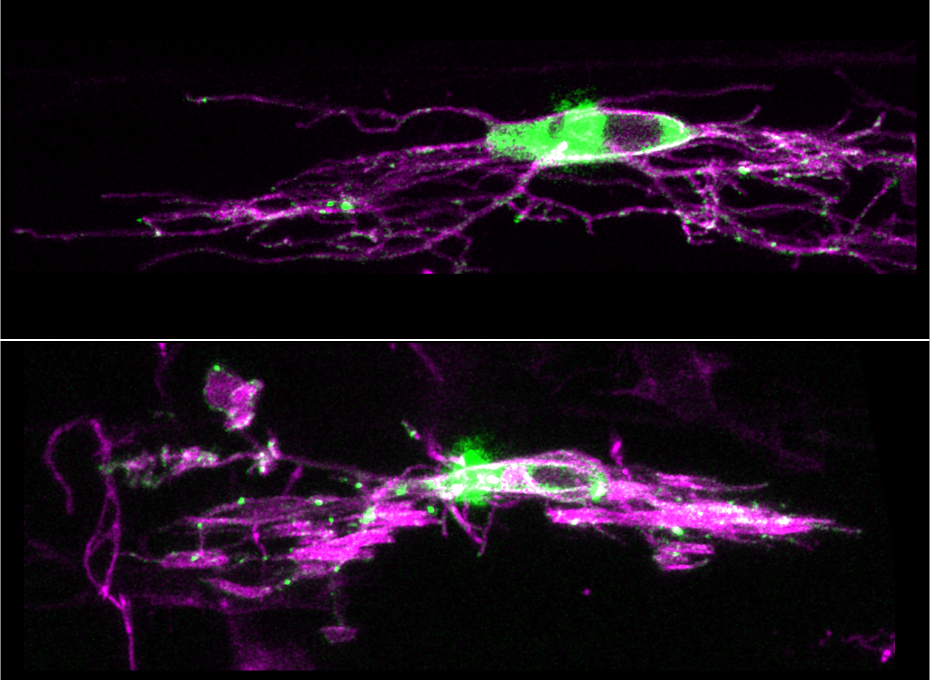
New research from the Vollum Institute for the first time reveals the function of a little-understood junction between cells in the brain that could have important treatment implications for conditions ranging from multiple sclerosis to Alzheimer’s disease to a type of brain cancer known as glioma.
Read the full article
The study published today in the journal Nature Neuroscience
Monk Lab
Study reveals structure of crucial receptor in brain development, function

Vollum scientists have revealed the molecular structure of a type of receptor that’s crucial to brain development and function.
Known as type A GABA receptors, these receptors are already targeted by pharmaceutical anesthetics, sedatives and antidepressants because of their important role in brain function. The discovery, published today in the journal Nature, reveals the dominant assemblies and states of the GABA receptor, a finding that could enable the development of new compounds that more specifically target a range of medical disorders.
More news and accolades
- OHSU neuroscientist earns award to advance schizophrenia research
- A roadmap to more equitable graduate school admissions
- Congratulations to Vollum faculty Drs. Wyatt, Mishra, and Zhong for their promotions!
- OHSU neuroscientist receives prestigious McKnight Scholar Award
- Three Vollum Institute/OHSU NGP graduate students named 2023 Lacroute Fellows
Recognition for our early career awardees
Graduate students and postdoctoral fellows are usually supported by research grants to individual faculty or by institutional training grants from the NIH. However, a sought-after perk for trainees is to obtain an individual fellowship from federal sources or foundations. Such awards are an honor and also provide important financial support for the trainee and their lab. Graduate students and postdoctoral fellows in the Vollum Institute have been remarkably successful in obtaining these awards over the past few years. This is a credit to the quality of the trainees and the support they receive from their mentors. Congratulations to all.
The Lacroute Fellows Program invests in School of Medicine graduate education, by supporting exceptional students performing innovative research in the Vollum/OHSU Neuroscience Graduate Program.
Congratulations to the 2023 fellows:
- Yessica Santana Agreda, Wright Lab
- Arielle Isakharov, Wright Lab
- Elizabeth Rose, Unni Lab
These 1-year fellowships cover $24,000 of the student’s stipend and provide an allowance of $1,000 for related expenses, such as attending scientific conferences or courses.
Cody Call, Ph.D., Monk Lab
NINDS F32: “Regulation of node of Ranvier formation and maintenance by astrocytes.”
Alejandra Fernandez, Ph.D., Wright Lab
Collins Medical Trust: “The role of Pten signaling in the intrinsic control of somatosensory neuron diversification.”
Kevin Guttenplan, Ph.D., Freeman Lab
Helen Hay Whitney Foundation: “How do astrocytes regulate neuronal circuits?”
Dongeun Heo, Ph.D., Monk/Freeman Labs
NINDS F32: "Investigating the role of diazepam binding inhibitor (DBI) in astrocytes and neural circuit maturation."
Taylor Jay, Ph.D., Freeman Lab
NINDS K99/ROO: "Investigating novel mechanisms that underlie glial-mediated synapse elimination in development and aging."
Yunsik Kang, Ph.D., Freeman Lab
NINDS K99/ROO Pathway to Independence: “How do astrocytes remodel neuronal circuits?”
Alex Nevue, Ph.D., Saunders Lab
BRAIN Initiative/NINDS F32: "Postnatal experience shapes gene expression and connectivity development in the cortex."
Cathy Spangler, Ph.D., Gouaux Lab
NIH Natl Cancer Inst: “Structural and functional characterization of native AMPA receptor complexes in glioblastoma.”
Dennis Weingarten, Ph.D., Jackman Lab
Grass Fellowship at MBL: "Sour patch: Electrophysiological study of synaptic transmission during hypercapnia in the lamprey.”
Landon Bayless-Edwards, Mao Lab
NIDA NRSA F30: "Intracellular signaling mechanisms underlying opioid modulation of pain"
Danica Bojovic, von Gersdorff and Mishra Labs
America Heart Association predoctoral fellowship: "Astrocyte gap junctions modulate neurovascular responses"
Hannah Collins, Monk and Emery Labs
NINDS F31: “Control of CNS Myelination by the E3 Ligase Component Fbxw7”
Rachel de la Torre, Freeman Lab
NINDS F31: “How do glia remodel the nervous system?”
Makayla Freitas, Gouaux Lab
National Science Foundation, Graduate Research Fellowship
Alexandra Houser, Baconguis Lab
National Science Foundation, Graduate Research Fellowship
Arielle Isakharov, Wright Lab
NEI F31 Predoctoral Fellowship: "Genetic analysis of the Robo3+ glycinergic amacrine cell"
Jennifer Jenks, Emery Lab
National Science Foundation, Graduate Research Fellowship
Omar Koita, Williams Lab
NINDS F99: “Mechanistic description of tolerance/withdrawal from opioids in the paraventricular nucleus of the thalamus.”
Tania Miramontes, Monk Lab
NINDS F31: "Investigating the role of cannabinoid receptors in oligodendrocyte development"
Yessica Santana Agreda, Wright Lab
HHMI Gilliam Fellow: "Transcriptional Control of Starburst Amacrine Cell Specification and Maturation"
Congratulations to all of our graduate researchers in the Vollum/OHSU Neuroscience Graduate Program who received ARCS Foundation Scholar Awards from the ARCS Oregon Chapter!
First Year: Milana Krush and Jed Syrenne
Second Year: Teva Bracha and Kim Engeln
Third Year: Sweta Adhikary, Amelia Culp, Makayla Freitas and Sierra Smith
Learn more about these scholars and the ARCS Foundation Oregon
Congratulations to the Neuroscience Graduate Program researchers — Ali Pincus, Prashant Rao and Petra Richer — who received 2020 N.L. Tartar Trust Fellowships. The $2,000 grants are awarded annually by the OHSU School of Medicine as a means to support research endeavors and career development. Keep up the great work!