Biomedical Innovation Program: From Academia to the Marketplace

About the Biomedical Innovation Program
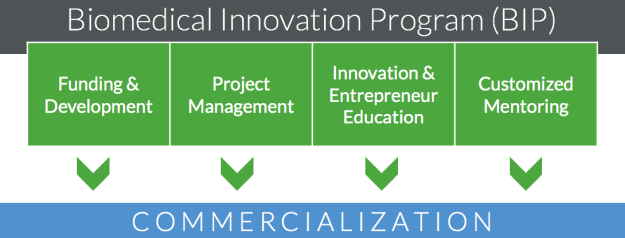
The Biomedical Innovation Program (BIP) at OHSU accelerates the delivery of healthcare technologies in order to improve human health. The program cultivates, evaluates, and funds promising translational projects with the objective of moving innovative technologies to clinical application through commercialization. Review program services in the diagram below or read about program services in this pdf.
BIP funding opportunities
Do you have an idea for a technical innovation to improve human health? Review the funding opportunities below, choose your focus, and reach out to us.
Track 1: Device, diagnostic, and software
This funding opportunity is intended for medical device and diagnostic technology projects. Through this funding mechanism, OCTRI, and OHSU Innovates, intend to support and accelerate creative, interdisciplinary device, diagnostic, and software development research at OHSU. Projects will typically be supported for a one-year period. Predetermined milestones and quantitative metrics of success will be evaluated on a regular basis.
Deadline: CLOSED
Amount: Up to $40,000
Eligibility: Principal Investigators must meet the OHSU eligibility requirements.
Track 2: Drug discovery and therapeutics development
This funding opportunity is intended for drug discovery platforms and therapeutic technology projects. Examples of responsive application topics include but are not limited to: development and validation of drug targets, screening platforms, small molecules, antibodies, vaccines and biologics. Through this funding mechanism, OCTRI, and OHSU Innovates, intend to support and accelerate creative, interdisciplinary drug discovery and therapeutic development research at OHSU. Projects will typically be supported for a one-year period. Predetermined milestones and quantitative metrics of success will be evaluated on a regular basis.
Deadline: CLOSED
Amount: Up to $60,000
Eligibility: Principal Investigators must meet the OHSU eligibility requirements.
Track 3: Digital health
This track of funding is intended to support novel and innovative ideas that use digital technology to improve healthcare delivery or advance biomedical research with the potential for commercialization, achieving cost savings, improving research efficiency, improving quality of patient care, and/or reducing provider burnout.
Grants will be awarded on a competitive basis, with budgets not to exceed $30,000. Additionally, each awardee will be assigned a project manager and a mentor to advise and help accelerate their project towards commercialization. Projects will typically be supported for a 6-month period; predetermined milestones and quantitative metrics of success will be evaluated on a regular basis.
Deadline: CLOSED
Amount: Up to $30,000
Eligibility: OHSU employees and students.
BIP application resources
Need help preparing your BIP application? Check out these vetted resources. Please note: the links and resources provided below are not meant to comprehensive, but rather to give applicants an introduction to concepts and terminology which may be unfamiliar.
General information
There are numerous online marketing and commercialization (introducing a new product or service into the market) resources. The following websites provide well-curated, concise articles and videos covering topics that are generally suited for beginners to the commercialization process.
- Stanford Biodesign resources and video (website)
- BPlans YouTube Channel
- Need assistance with searching the literature for your BIP application? Get support from the OHSU Library
Market Research Lab: Intelligence Research with GlobalData
Need assistance with market research for your technology or idea? Survey the competitive landscape with GlobalData Medical. Schedule an appointment with OHSU's Market Research Lab.
Market analysis documents
Marketing and stakeholder analyses help identify key competition, opportunities, potential investors, and the economic environment within your innovation’s specific industry.
Value Proposition and the business model canvas
Creating a value proposition and populating a business model canvas allows you to identify the problem and solution, determine who will benefit, and predict the value of the proposed technology over existing solutions.
Intellectual property
Intellectual property protects original ideas and innovations typically through copyrights, patents, trademarks, and trade secrets. OHSU Technology Transfer has highly-trained Patent Associates and Technology Development Managers who are available to help you navigate the complex intellectual property environment.
- Intellectual Property 101 (website)
- Seven step strategy from the United States Patent and Trademark Office (website)
- Search and read the full text of patents from around the world with Google Patents
Regulatory
Depending on the innovation, specific regulatory pathways must be followed to ensure adherence to federal guidelines (e.g. FDA). Inventors should have a general understanding of the regulatory compliance requirements related to their innovation.
- Regulatory Basics from the Stanford Biodesign program (video)
Elevator pitch and pitch deck
Developing effective elevator pitches and slide decks are essential tools for promoting your technology and attracting investors in a concise, clear, and engaging manner.
- Scientists, Here’s the Best Elevator Pitch to Sell Yourself to a Potential Employer (article)
- The 7 Key Components of a Perfect Elevator Pitch (article & video):
- How to Develop a PowerPoint Pitch Deck for Biotech Investor Presentations (article)
Exit strategy
Identifying clear commercialization pathways is important for the success of a potential technology, whether it be determining licensing potential, startup opportunities, or pursuing adequate intellectual property protections to ensure maximum success.
- From Lab to Market: Pitt Commercialization Process (website)
Non-confidential summary
A non-confidential summary (NCS) serves as a technology advertisement to attract potential licensees, investors, or other business partners without compromising key intellectual property.
- Non-Confidential summary example and template (doc)
- Tips from OHSU Technology Transfer on crafting an NCS (pdf)
Attention entrepreneurs! Thinking of forming a start-up company? OHSU Collaborations & Entrepreneurship - New Ventures has you covered.
Additional information
Program partners
The Biomedical Innovation Program is a partnership between OCTRI, OHSU Technology Transfer, and OHSU Collaboration and Entrepreneurship. Additional funding for the program has been provided by:
Learn more about resources in OHSU Technology Transfer that can help you commercialize your research.
Past BIP-funded projects
Explore past BIP-funded projects and award recipients here.

Questions about the BIP?
Reach out to us.
Jonathan Jubera, M.B.A
Project Manager
jubera@ohsu.edu
Aditi Martin, Ph.D.
Program Director
martiad@ohsu.edu

Check out the outcomes report for the OCTRI Innovation and Pilots Awards team

Market Research Lab: Intelligence Research with GlobalData
Need assistance with market research for your technology or idea?
Schedule an appointment with OHSU's Market Research Lab.

Looking for more opportunities?
Check out our Innovation and Entrepreneur Education web page.
Quick list of OCTRI grant numbers
CTSA award: UL1TR002369
TL1 program: TL1TR002371
KL2 program: KL2TR002370