Mishra Lab
Lab overview
Astrocytes in neurovascular coupling
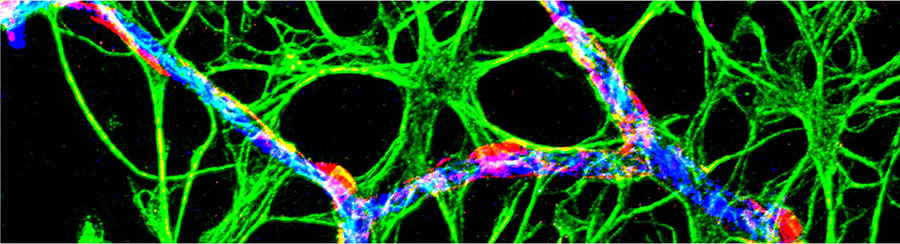
Glial cells called astrocytes are abundant in the central nervous system. Increasingly, scientists are recognizing important contributions of astrocytes to many aspects of CNS function, one of them being neurovascular coupling – the process by which active neurons signal to blood vessels to increase the local blood flow and hence the supply of energy substrates. This process underlies several non-invasive neuroimaging techniques applied to human cognitive research and clinical diagnosis, such as functional magnetic resonance imaging. Under healthy mature conditions, neurovascular coupling is very robust, thus allowing this proxy measure to reliably report neuronal activity. However, the neurovascular coupling relationship is altered in pathological/disease contexts, which not only results in a mismatch of energy supply and demand in the brain, but also complicates the interpretation of neuroimaging data. Work in the Mishra lab is aimed at understanding the mechanisms of neurovascular coupling impairment in disease, particularly at the microvascular capillary level, with a focus on the role of astrocytes.
Neurovascular coupling impairment following stroke
A significant attenuation of neurovascular coupling is observed clinically in stroke patients for many years after the stroke. In the Mishra lab, we have revealed a similar suppression of neurovascular coupling at capillaries after experimental stroke models. This impairment occurs in peri-infarct tissue that otherwise looks healthy but displays changes in astrocyte morphology and expression patterns suggesting reactive astrogliosis. The functional outcomes manifested by these astrocytic changes are not understood. Current work in Dr. Mishra’s lab investigates the possibility that the constriction of capillaries and loss of neurovascular coupling observed after stroke may be due to pathophysiological signaling from reactive astrocytes. Some of the characteristics of these reactive astrocytes are reminiscent of developing astrocytes; thus, we are also studying how the neurovascular coupling response matures with the hope that this may help us understand how it is dysregulated after stroke.
Chronic effects of stroke-induced neurovascular impairment – role in dementia
Astrogliosis and neurovascular impairment are common features of many diseases of the central nervous system (including Alzheimer's disease, traumatic brain injury, vascular dementia, and chronic hypertension). Especially noteworthy are the epidemiological observations that patients harboring ischemic injuries develop dementia at a higher rate, and conversely, patients with dementia show evidence of ischemic damage in their brains. Thus, we are also investigating the long-lasting effects of mild strokes on neurovascular coupling and their contributions to cognitive loss.
Functions of astrocytic gap junctions
In a more basic research direction, we are also interested in how gap junctions help astrocytes do what they do. Although it has been known for many decades that astrocytes are coupled by gap junctions, the role of this coupling in neurovascular coupling, a prominent function of astrocytes, remains unknown. Thus, we are investigating whether gap junctional coupling of astrocytes contributes to their functions in K+ buffering and neurovascular coupling.
Lab members


Anusha Mishra, Ph.D.
Principal Investigator
Anusha is interested in the physiological and pathological interactions between astrocytes and the cerebral microvasculature. Her interest in astrocytes began with the visualization of their fine ultrastructure while studying presynaptic plasticity using electron microscopy in Kristen Harris’s lab. She did her graduate work with Eric Newman at the University of Minnesota investigating astrocyte regulation of retinal vasculature in healthy and disease conditions. She then worked with David Attwell at University College London, where she demonstrated that astrocytes regulate capillary diameter in the cerebral cortex, and found evidence suggesting that ischemia-induced capillary constriction might underlie the no-reflow phenomenon following stroke. She joined OHSU in November 2016 and moved to the Jungers Center as an Assistant Professor in 2019. Her research focuses on astrocyte-dependent mechanisms of neurovascular coupling during development and following ischemic stroke.
Anusha also serves as a faculty member for the Neuroscience Graduate Program and the D3 hub of Graduate Program in Biomedical Sciences. She is also an active participant in the Department of Neurology DEI meetings.
Outside the lab, Anusha enjoys cooking, hiking, camping and reading fiction.

Teresa Stackhouse, B.A.
Graduate Student
Teresa received her B.A. in Biology from Lewis & Clark College in 2016, where she studied the role of phosphorylation on alpha-synuclein aggregation at the synapse in the lab of Dr. Tamily Weissman. Upon graduation, Teresa joined the Unni Lab at OHSU as a Research Assistant, where she continued studying mechanisms of alpha-synuclein aggregation until joining the Neuroscience Graduate Program at the Vollum Institute in 2018.
In the fall of 2019, Teresa joined the Mishra lab as a graduate student. Her major research interests include astrocyte biology and the role of glia in development and disease. Teresa is enthusiastic to combine these interests by studying common features of developing and reactive astrocytes and how this influences neurovascular coupling. Outside the lab, Teresa is passionate about broadening access to STEM education, particularly at the graduate and early career levels. She has been involved in Women in Science Portland and Alliance for Visible Diversity in Science at OHSU for a number of years.

Danica Bojovic, B.A.
Graduate Student
Danica got her B.A. from Grinnell College, where she studied Biology and French. In the spirit of liberal arts education, she did several smaller research projects in neuroscience, psychology, and movement sciences. In neurosciences, she participated in two different projects, one studying the mechanism of homocysteine-induced exacerbation of oxidative stress in the mouse neuromuscular junction and the other studying brain atrophy in post-mortem brain tissues from Multiple Sclerosis patients. During her undergraduate studies, she became interested in glial cells and continued her graduate education in the Neuroscience Graduate Program at OHSU, studying astrocytes under a collaboration between the Mishra and von Gersdorff labs. Her thesis project focuses on the role of astrocyte gap junctions in potassium uptake and neurovascular coupling.
Outside of lab, Danica likes to cook, study languages, and socialize. She's interested in community building and promoting diversity in science. She is serving as Vice Chair of Administration at International Employee Resource Group (IERG) at OHSU.
Ben Zimmerman, Ph.D.
Postdoctoral Scholar
Ben is a postdoctoral researcher interested in the role of the brain’s vascular health in age-related cognitive decline. In the lab, he is currently studying the effects of the medicinal herb, Centella asiatica, which induces anti-oxidant pathways, on brain vessels. Ben holds an R90 BRIDG fellowship from the National University of Natural Medicine, Portland OR, where he spends part of his time contributing to projects investigating the vascular effects of yoga pranayama techniques.
Ben completed his PhD in neuroscience at the University of Illinois at Urbana-Champaign, where he stayed as a postdoc supported by a Beckman Fellowship at the Beckman Institute for Advanced Science and Technology. There he did graduate work under the primary mentorship of Monica Fabiani and Gabriele Gratton with support from an NSF IGERT in Neuroengineering. He researched the role of vascular health in normal aging using a variety of human neuroimaging techniques. During his postdoc training, he expanded this research to include a multidisciplinary team of other mentors at the University of Illinois, including Brad Sutton, Fatima Husain, Yuliy Baryshnikov, and John Rogers.
He also did research at OHSU with Bill Rooney and Jacob Raber with support from a Burroughs Wellcome Fund collaborative travel grant. This expansive, interdisciplinary background has instilled in him a passion for translational, team-science.
During his training, he has served as an editorial assistant for the journal, Psychophysiology, and currently serves on the diversity and outreach committee for the Society of Psychophysiological Research. He loves mentoring and teaching, and has taught courses on the psychology of aging, statistics, and seminars on neuroimaging techniques.

Simone Woodruff, B.Sc.
MD-PhD Student
Simone received a B.Sc. in Neuroscience from the University of California, Riverside in 2019. She was an undergraduate researcher in Dr. Iryna Ethell’s lab from 2015 to 2020, during which time she received the Undergraduate Research Mini-Grant and Chancellor’s Research Fellowship. In Dr. Ethell’s lab, she investigated the role of astrocytic ephrin-B1 in synapse regulation in the hippocampus. This is where she developed her desire to pursue research, in addition to medicine. She joined the MD-PhD Program at OHSU in 2020 and aspires to have a career in neurology and neuroscience.
Simone joined the Mishra lab in 2022, where she is studying the connection between stroke and dementia. She is performing a cross-sectional study to determine how ischemic events affect cerebrovascular function over time and how this alters the progression of behavioral/cognitive deficits in Alzheimer’s disease.
Outside of school and work, Simone enjoys dancing, playing video games, and going on road trips. She is involved in student organizations at OHSU, including Alliance for Visible Diversity in Science, American Women’s Medical Association, and Students Offering Support.

Laura M. Knittel, Ph.D.
Research Assistant
Laura earned her Ph.D. in Neuroscience from OHSU in 2002, where she studied the role of steroid hormones during remodeling of the nervous system during metamorphosis of the moth, Manduca sexta, in Dr. Karla Kent’s laboratory. She went on to do a Postdoctoral Fellowship in the laboratory of Dr. Philip Copenhaver at OHSU studying the directed migration of a population of neurons during Manduca’s embryonic development. In 2005, Laura left academics in order to focus on raising her two daughters full time. Now that they are grown, she has returned to the workforce, and is excited to be using her skills again!

Raja Estes, B.S.
Graduate Student
Raja got her B.S. from the University of Washington where she studied Biology with a Physiology focus. There she started her journey in neuroscience in Dr. Matthew Kaeberlein’s lab studying a link between aging and onset of Alzheimer's Diseases by performing high throughput drug screenings on C.elegans. Upon graduation she continued her interest in neurodegenerative diseases in Dr. Marie Davis’s lab at the Seattle Veteran’s Association Hospital, where she established astrocyte cultures derived from human Induced Puripotent stem cells isolated from Parkinson's Disease patients. This work supported the investigation of the role of the gene GBA in the spread of alpha-synuclein from neurons to astrocytes. Motivated by this new interest in astrocytes, she joined the Monk and Mishra Labs as a co-mentored student in 2023, where she is investigating the development of astrocyte endfeet, specialized processes that interface the vasculature of the brain.
Outside of the lab, Raja is a mentor for minority students in science, is actively involved with the Graduate Research Union as an e-board member, as well as the Alliance for Visible Diversity in Science as a steward member. She is committed to advocating for an inclusive and safe space for all students to feel welcome on campus. While not on campus, Raja enjoys hiking the Oregon wilderness, biking, fashion and makeup, and cooking with friends.

Bradley Marxmiller, B.A.
Research Assistant
Bradley graduated from Portland State University with a B.A. in Biology and a Neuroscience minor. As an undergraduate NIH BUILD EXITO scholar, he performed research on the role of prefrontal cortex interneuron subtypes during decision making at the Abbas Lab at OHSU/VA. His interest in Vasoactive Intestinal Peptide interneurons and their interactions with astrocytes lead him to have an interest in glia, neurovascular coupling, and the Mishra Lab.
The main focus of Bradley’s research in the Mishra Lab is on the signaling pathways that underlie capillary contraction. Capillaries contribute to cortical blood flow regulation during health and dysregulation of these pathways play important roles during diseased states. Using a promising new genetic system that exclusively targets brain pericytes, he hopes to elucidate the intracellular mechanisms that control pericyte contractility and, therefore, blood flow in the central nervous system.
Outside of the lab, Bradley spends his time reading fantasy novels, volunteering with NW NOGGIN, and playing rugby with the Portland Lumberjacks.
Projects
Mechanisms of neurovascular coupling in development and disease
The brain requires high levels of oxygen and glucose for optimal function, but does not have significant metabolic substrate storage within its tissue. Increases in local neural activity trigger changes in cerebral blood flow to supply this increased energy demand. This process is termed neurovascular coupling is partly mediated by the release of vasoactive molecules from astrocytes in a Ca2+-dependent manner.
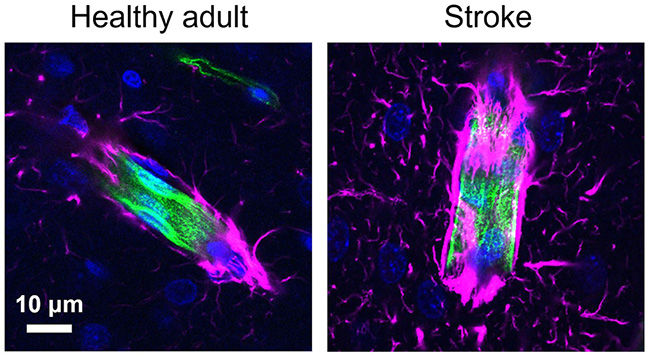
In healthy adult brains, neurovascular coupling leads to vascular dilation and increase in blood flow, which is reflected in human neuroimaging studies as an increase in blood oxygen level-dependent signals; however, in young rodents and humans, this response is often absent or negative. This early negative neurovascular coupling has important implications for brain development, but its mechanism is not understood.
Many neurological disorders are also associated with loss of neurovascular coupling, which can result in chronic metabolic deprivation and contribute to further neuronal dysfunction. In the case of ischemic stroke, loss or attenuation of neurovascular coupling is commonly observed in patients, and the Mishra lab has observed a similar loss in a rodent model of transient ischemic stroke. The mechanism for this loss of neurovascular coupling is also unknown.
We are using ex vivo neurovascular coupling assays and calcium imaging to study both these conditions to understand mechanisms regulating neurovascular coupling with a particular focus on the role of metabotropic glutamate receptor 5, which is expressed in young astrocytes and re-induced in reactive astrocytes.
Project lead: Teresa Stackhouse, assisted by Laura Knittel
Contribution of stroke-induced cerebrovascular impairments to cognitive dysfunction and dementia
As life expectancy of the global population has increased, so has the incidence of Alzheimer’s disease and other dementias. However, there are still no effective treatments for the disease. Mounting research points towards a reduction in cerebral blood flow as one of the earliest changes that occurs in the brains of those who develop Alzheimer’s disease and other related forms of dementia.
A significant risk factor for dementia is a history of ischemic injuries. Patients who experience strokes or transient ischemic attacks have a higher incidence of dementia. Conversely, a significant proportion of dementia patients show signs of silent or micro-infarcts, indicating brief blockages of blood flow to regions of the brain. As ischemic strokes can induce cerebral blood flow dysregulation, these data hint at ischemia acting as a triggering factor in dementia.
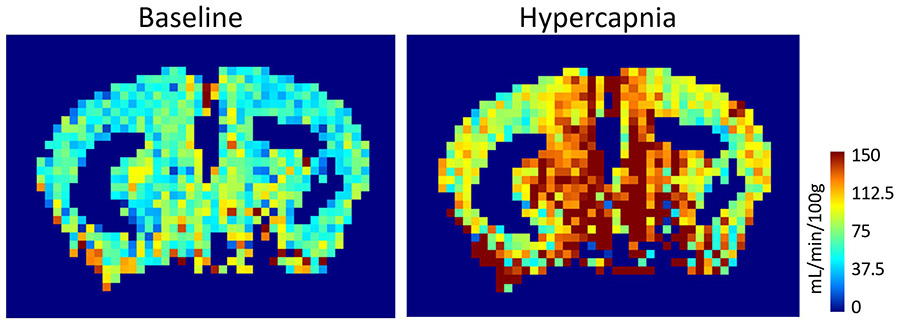
We are studying the long-term consequences of ischemia in an Alzheimer’s-like transgenic mouse model using a variety of techniques. Behavioral assays are used to study cognition, magnetic resonance imaging to measure resting blood flow and vascular reactivity, laser speckle contrast imaging and laser Doppler flowmetry to quantify neurovascular coupling in vivo, acute brain slices to assess neurovascular coupling ex vivo, and immunohistological stains are used to identify pathophysiology. Findings from this project could shed light on how silent stroke triggers or accelerates features of Alzheimer’s disease.
Project lead: Simone Woodruff (collaboration with Dr. Martin Pike at the Advanced Imaging Research Center, OHSU and Dr. Laura Villasana at Legacy Research Institute)
Astrocyte gap junctions, potassium uptake, and neurovascular coupling
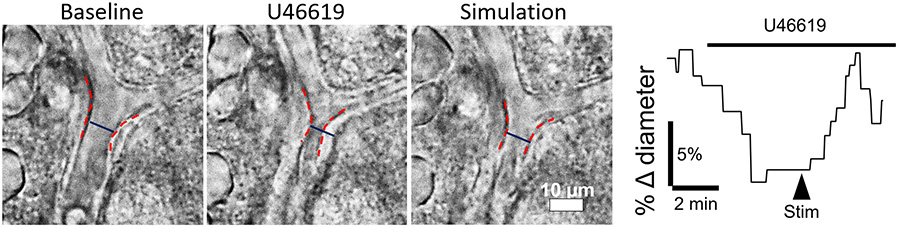
Astrocytes are coupled by gap junctions into a glial syncytium. Exactly how coupling between astrocytes helps them perform their critical functions remains unclear. In this project, we are interested in the contribution of astrocyte gap junctions in two key astrocyte functions: K+ buffering and neurovascular coupling. With their fine processes that concentrate around synapses and their endfeet that fully cover blood vessels in the brain, astrocytes are well poised to convey messages about neural activity to the vascular cells to induce neurovascular coupling.
At their peri-synaptic processes, astrocytes express neurotransmitters transporters as well as receptors and an abundance of the K+ channel Kir4.1. It is believed that astrocytes take up the K+ released during neuronal activity to prevent extracellular buildup and that the K+ can be buffered away to regions of low activity via gap junctions, thus maintaining neuronal extracellular homeostasis. However, evidence in support of this hypothesis is still murky. Furthermore, although it is known that K+ uptake can contribute to neurovascular coupling, whether gap junctional coupling of astrocytes modulates neurovascular coupling remains unknown. We are using ex vivo whole-cell patch clamping technique and vascular imaging to answer these questions.
Project lead: Danica Bojovic (co-mentored by Dr. Henrique von Gersdorff)
Cerebrovascular effects of Centella asiatica, a plant extract with antioxidant properties
The botanical, Centella asiatica, sometimes called Gotu Kola, has been used traditionally in Ayurvedic medicine to improve brain function and prevent cognitive decline. Recent research has added support for this use by finding promising results for the cognitive benefits of C. asiatica in mouse models of aging. However, the mechanisms underlying those benefits are still being elucidated.
In our lab, we are studying if C. asiatica can have direct effects on the tone of the microvasculature using ex vivo mouse brain slices, as a subproject within BENFRA. If it does, then C. asiatica might promote health in the brain by acutely increasing blood flow. We are testing different dosages of a C. asiatica water extract to determine if there is an ideal dose to elicit such a response. We are also testing the effects of different isolated molecular constituents of the whole herb to better understand what part of C. asiatica may cause these effects.
In separate experiments, we will also test if longer-term C. asiatica supplementation has protective effects against oxidative stress-induced changes in brain vessels. We are examining ex vivo brain slices from mice fed a diet including C. asiatica over several weeks vs. mice who were fed a normal diet, and examining whether C. asiatica treatment reduces vascular response to oxidative stress.
Identifying the mechanisms by which C. asiatica is beneficial will give us a deeper understanding of when and how to use it as a supplement or therapy. It will also shed light on the role of oxidative stress on vascular aging. Furthermore, understanding the specific chemical constituents that are responsible for those mechanisms may also be useful in developing new future therapies.
Project lead: Ben Zimmerman (collaboration with Dr. Amala Soumyanath at the BENFRA Center and Dr. Martin Pike and Dr. Manoj Sammi at the Advanced Imaging Research Center)
Intracellular mechanisms underlying pericyte contractility
A dense network of capillaries makes up the majority of the estimated 300 miles of microvasculature in the human brain. The location, morphology, and physical properties of these small vessels make them uniquely situated to control cerebral blood flow. To date, the Mishra Lab and others have shown that pericytes (cells that line and wrap around capillaries) have a large role in regulating healthy blood flow and are amongst the first cells to react to metabolic conditions that trigger neurovascular coupling. In pathological situations, such as post-stroke and in Alzheimer’s Disease and Related Dementias (AD/ADRD), increased contraction of pericytes has been associated with reduced blood perfusion, potentially leading to neural cell loss and cognitive decline. However, despite the importance of these cells in cerebral hemodynamics in both healthy and disease states, the intracellular mechanisms that drive pericyte contractility are as yet unknown.
Using a newly engineered genetic system (developed by Dr. Zhen Zhao’s lab at USC) to exclusively target brain pericytes, the Mishra Lab is examining cellular signaling cascades involved in pericyte contractility.
Project Lead: Bradley Marxmiller (in collaboration with Dr. Andy Shih at the Seattle Children's Research Institute and Dr. Dimitrios Davalos at the Cleveland Clinic)
Investigating astrocyte endfoot development in zebrafish and rodents
Astrocytes are the most abundant glial cell type in the brain, directly supporting neuronal circuit function. Their fine processes extend around synapses and endfeet processes wrap around vasculature. These astrocyte endfeet cover over most of the mammalian cerebral vasculature where they are a major component of the neurovascular unit, which regulates blood flow in response to neuronal demand and supports the blood brain barrier. However, there is little known about how astrocyte endfeet form and how they interact with new, continually growing blood vessels that are developing at the same time as endfeet. This project aims to answer these questions through the investigation of astrocyte development in zebrafish and rodents.
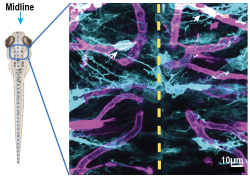
To visualize the development of astrocyte endfeet, we are collaborating with Kelly Monk’s lab and employing the zebrafish. These fish are a powerful vertebrate model organism because they are transparent during their larval development and offer a suite of genetic tools. This allows us to live image astrocyte endfoot development in vivo alongside vascular development in the same larval zebrafish. We complement this dataset by assessing astrocyte endfeet formation in rodents. We are also investigating the mechanisms underlying astrocyte endfeet morphogenesis.
Project Lead: Raja Estes (collaboration with Dr. Kelly Monk at the Vollum Institute)
Publications
Selected publications
2024
Mishra, A., Gordon, G. R., MacVicar, B. A., & Newman, E. A. (2024). Astrocyte Regulation of Cerebral Blood Flow in Health and Disease. Cold Spring Harbor perspectives in biology, a041354. Advance online publication. https://doi.org/10.1101/cshperspect.a041354
2023
Iadecola C, Smith EE, Anrather J, Gu C, Mishra A, Misra S, Perez-Pinzon MA, Shih AY, Sorond FA, van Veluw SJ, Wellington CL; on behalf of the American Heart Association Stroke Council; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Radiology and Intervention; Council on Hypertension; and Council on Lifestyle and Cardiometabolic Health. (2023) The neurovasculome: key roles in brain health and cognitive impairment: a scientific statement from the American Heart Association/American Stroke Association. Stroke. doi.org/10.1161/STR.0000000000000431
Diaz-Castro B, Robel S and Mishra A. (2023) Astrocyte endfeet in brain function and pathology: fascinating open questions. Annual Review of Neuroscience. doi: 10.1146/annurev-neuro-091922-031205
2022
Shinto LH, Raber J, Mishra A, Roese N and Silbert LC. (2022) A Review of Oxylipins in Alzheimer’s Disease and Related Dementias (ADRD): Potential Therapeutic Targets for the Modulation of Vascular Tone and Inflammation. Metabolites. 12:826. doi.org/10.3390/metabo12090826
Kaul S, Methner C, Cao Z, Mishra A. (2022) Mechanisms of the "No-Reflow" Phenomenon After Acute Myocardial Infarction: Potential Role of Pericytes. JACC Basic Transl Sci. 8:204. doi: 10.1016/j.jacbts.2022.06.008
McConnell HL and Mishra A. (2022) Cells of the Blood-brain Barrier: an Overview of the Neurovascular Unit in Health and Disease. Methods Mol Biol. 2492:3-24. doi: 10.1007/978-1-0716-2289-6_1
Bojovic D, Stackhouse TL and Mishra A. (2022) Assaying activity-dependent arteriole and capillary responses in brain slices. Neurophotonics. 9:031913. doi.org/10.1117/1.NPh.9.3.031913
Nizari S, Hawkes C, Hattori Y, Mishra A. (2022) Editorial: The Neurovascular Unit as a Potential Biomarker and Therapeutic Target in Cerebrovascular Disease. Frontiers in Aging Neuroscience. 14:908716. doi: 10.3389/fnagi.2022.908716
2021
Li Z, McConnell HL, Stackhouse TL, Pike MM, Zhang W, and Mishra A. (2021) Increased 20-HETE signaling suppresses capillary neurovascular coupling after ischemic stroke in regions beyond the infarct. Frontiers in Cellular Neuroscience. 15:762843. doi: 10.3389/fncel.2021.762843.
Methner C, Cao Z, Mishra A and Kaul S. (2021) Mechanism and Potential Treatment of the ‘No Reflow’ Phenomenon after Acute Myocardial Infarction: Role of Pericytes and GPR39. AJP Heart and Circulatory Physiology. 321(6):H1030-H1041. doi: 10.1152/ajpheart.00312.2021. (chosen for APS Select, December 2021)
Stackhouse TL and Mishra A. (2021) Neurovascular coupling in development and disease: focus on astrocytes. Frontiers in Cell and Development Biology. 9:702832. doi: 10.3389/fcell.2021.702832
Thornton CA, Mulqueen RM, Torkenczy KA, Nishida A, Lowenstein EG, Fields AJ, Steemers FJ, Zhang W, McConnell H, Woltjer RL, Mishra A, Wright KM and Adey AC. (2021) Spatially-mapped single-cell chromatin accessibility. Nature Communications 12, Article number: 1274.
Escartin C*, Galea E*, Lakatos A, O’Callaghan JP, Petzold GC, Serrano-Pozo A, Steinhauser C, Volterra A, Carmignoto G, Agarwal A, Allen NJ, Araque A, Barbeito L, Barzilai A, Bergles DE, Bonvento G, Butt AM, Chen WT, Cohen-Salmon M, Cunningham C, Deneen B, De Strooper B, Díaz-Castro B, Farina C, Freeman M, Gallo V, Goldman JE, Goldman SA, Götz M, Gutiérrez A, Haydon PG, Heiland DH, Hol EM, Holt MG, Iino M, Kastanenka KV, Kettenmann H, Khakh BS, Koizumi S, Lee CJ, Liddelow SA, MacVicar BA, Magistretti P, Messing A, Mishra A, Molofsky AV, Murai K, Norris CM, Okada S, Oliet SHR, Oliveira JF, Panatier A, Parpura V, Pekna M, Pekny M, Pellerin L, Perea G, Pérez-Nievas BG, Pfrieger FW, Poskanzer KA, Quintana FJ, Ransohoff RM, Riquelme-Perez M, Robel S, Rose CR, Rothstein J, Rouach N, Rowitch DH, Semyanov A, Sirko S, Sontheimer H, Swanson RA, Vitorica J, Wanner IB, Wood LB, Wu J, Zheng B, Zimmer ER, Zorec R, Sofroniew MV*, and Verkhratsky A*. (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nature Neuroscience 24, 312–325.
Mishra A, Hall CN, Howarth CH and Freeman RD. (2021) Key relationships between non-invasive functional neuroimaging and the underlying neuronal activity. Philosophical Transactions of the Royal Society London B Biological Sciences. 376(1815):20190622. doi: 10.1098/rstb.2019.0622.
*Howarth CH, *Mishra A. and *Hall CN. (2021) More than just summed neuronal activity: how multiple cell types shape the BOLD response. Philos Trans R Soc Lond B Biol Sci. 376(1815):20190630.
2019
*McConnell HL, *Li Z, Woltjer RL and Mishra A. (2019) Astrogliosis and cerebrovascular dysfunction in neurological disorders: correlation or causation? Neurochemistry International; 128:70-84.
Nortley R, *Korte N, *Izquierdo P, *Hirunpattarasilp C, *Mishra A, *Jaunmuktane Z, *Kyrargyri V, Pfeiffer T, Khennouf L, Madry C, Gong H, Richard-Loendt A, Huang W, Saito T, Saido TC, Brandner S, Sethi H, Attwell D. (2019) Amyloid beta oligomers constrict human capillaries in Alzheimer's disease via signalling to pericytes. Science; 365:eaav9518.
Methner C, Mishra A, Golgotiu K, Li Y, Wei W, Yanez ND, Zlokovic B, Wang RK, Alkayed NJ, Kaul S and Iliff JJ. (2019) Pericyte constriction underlies capillary de-recruitment during hyperemia in the setting of noncritical arterial stenosis. AJP Heart and Circulation Physiology; 317:H255-H263.
2017
Mishra A. (2017) Binaural blood flow control by astrocytes: Listening to both synapses and the vasculature. Journal of Physiology; 595:1885-1902.
2016
Mishra A, Reynolds JP, Chen Y, Gourine AV, Rusakov DA and Attwell D. (2016) Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nature Neuroscience; 19:1619-27.
2015
Attwell D, Mishra A, Hall CN, O’Farrell F and Dalkara T. (2015) What is a pericyte? Journal of Cerebral Blood Flow and Metabolism; 36:451-5.
2014
*Hall CN, *Reynell C, *Gesslein B, *Hamilton NB, *Mishra A, Sutherland BA, O'Farrell FM, Buchan AM, Lauritzen M, Attwell D. (2014) Capillary pericytes regulate cerebral blood flow in health and disease. Nature; 508:55-60.
*Mishra A, *O’Farrell FM, Reynell C, Hamilton NB, Hall CN and Attwell D. (2014) Imaging pericytes and capillary diameter in brain slices and isolated retinae. Nature Protocols; 9:323-36.
2012
Mishra A and Newman EA. (2012) Aminoguanidine reverses the loss of flicker-induced vasodilation in a rat model of diabetic retinopathy. Frontiers in Neuroenergetics; 3:10.
2011
Mishra A, Hamid AA and Newman EA. (2011) Oxygen modulation of neurovascular coupling in the rat retina. Proceedings of the National Academy of Sciences USA; 108:17827-31.
2010
Mishra A and Newman EA. (2010) Inhibition of inducible nitric oxide synthase reverses the loss of functional hyperemia in diabetic retinopathy. Glia; 58:1996-2004
Lab photos

Lab alumni

Ozama (Oz) Ismail, Ph.D.
Postdoctoral Researcher, 2020-2022
Oz completed his PhD in neuroscience at University College London’s Centre for Advanced Biomedical Imaging, when he also did a short research placement in the Iliff Lab at OHSU. Prior to his PhD, he was the Centre’s Research Operations Manager and previously worked as a Research Technician in collaboration with Eli Lilly & Company and at the Wellcome Trust Sanger Institute in Cambridge within the Mouse Genetics Programme. In the Mishra lab, Oz examined the link between stroke and dementia. He studied chronic changes in cerebral blood flow regulation after ischemic events, with the goal of understanding how these changes contribute to the onset or acceleration of Alzheimer’s disease later in life.
Away from his day job, Oz is an avid science communicator and co-hosts a podcast called “Why Aren’t You A Doctor Yet?” He is also an avid advocate for diversity: he co-founded the Minorities in STEM network in the UK and sat on the board of the Alliance for Visible Diversity in Science at OHSU.
He is currently the Director of Scientific Programs, Alzheimer's Association®, see LinkedIn.

Heather McConnell, MCR, PhD
Postdoctoral fellow, 2018-2020
Heather received her PhD in neuroscience in 2018 and her master’s degree in clinical research in 2016. During her PhD research, she worked in the Neuwelt lab and used ultra-high field strength MRI to determine how viral-sized contrast agents interact with cells in cancerous brain lesions. Heather joined the Mishra lab as a post-doctoral fellow and investigated how reactive astrocytes in the brain might precipitate a mismatch between neuronal activity and cerebral blood flow after stroke. Heather introduced the use of arterial spin-labeling magnetic resonance imaging (ASL-MRI) to study cerebral blood flow in the Mishra lab and helped set up many projects, several of which are still in progress.
Heather is now working as a scientific writer and helps other scientists bring clarity and accuracy to their scientific and clinical communications.

Zhenzhou Li, MBBS
Visiting Scholar, 2017-2019
Zhenzhou obtained his MBBS degree from General Hospital of Ningxia Medical University, where he trained as an anesthesiologist. His interest in the biological underpinnings of how the brain works led him to look for research opportunities. With the support of a Western Light Talent Training Fellowship, Zhenzhou joined the Mishra lab to study aberrant signaling mechanisms of acute neurovascular coupling dysfunction after stroke.
Zhenzhou is now back at General Hospital of Ningxia Medical University, practicing as an anesthesiologist while simultaneously pursuing a PhD in Neuroscience.

Evan Calkins, B.S.
Evan was a research Assistant in the Mishra lab from early 2020 till late 2021. He assisted with several projects involving glial response and neurovascular coupling defects in stroke.