Institutional Review Board (IRB)
The OHSU Institutional Review Board (IRB) reviews research that involves human subjects. In general, a human subject is a living individual about whom an investigator conducting research obtains (1) data through intervention or interaction with the individual or (2) identifiable private information.
See About the IRB for more information on Human Subject Research and the role of the OHSU IRB.
OHSU members should visit the Institutional Review Board (IRB) page on O2.
News and announcements
Previously, protocol exceptions were submitted via modification to obtain prospective IRB review/approval. Sometimes, this path was problematic because another submission was already in process. The eIRB system only allows one modification of the type in process at a time. Oftentimes, these are urgent requests. Now, there is a new mechanism for these - they can be submitted as RNIs, even if there is a modification in process.
Steps to submit a Protocol Exception via RNI:
- Select 'New Protocol Exception' to open the RNI.
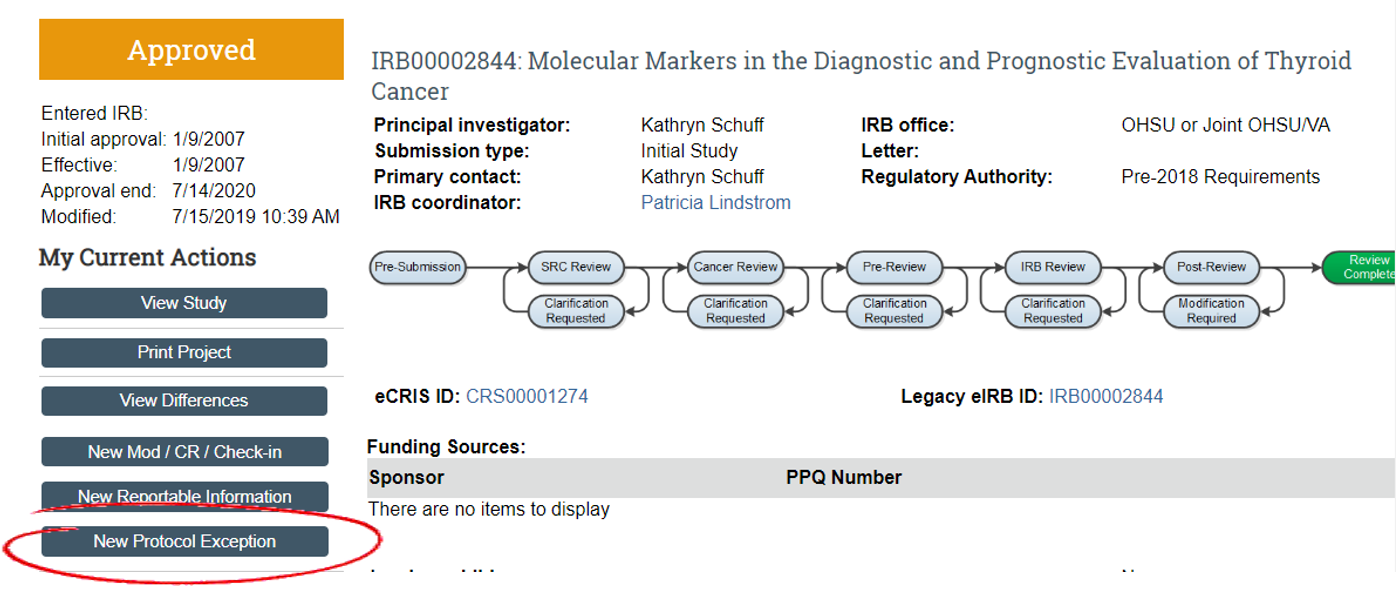
- The category list within the RNI form now includes 'Protocol Exception' as an option.

- Describe the need for the exception in question 7.
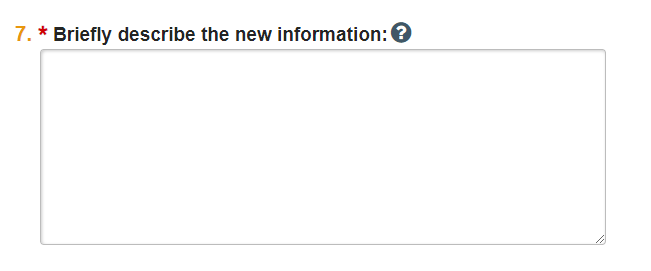
- Be sure to include information about how the protocol exception affects the subject, e.g., whether it places the subject at any risk but why the risk is acceptable or reduces risk to subject.
- Upload sponsor approval of the protocol exception, if applicable in question 14.

There is a new Continuing Review Form and Adverse Event Table for Continuing Review (template) to use for all future continuing reviews.
For the latest version of all our documents, please use our IRB Policies and Forms page.
Check out our Online IRB 101 Training!
You no longer need to register through compass or complete the IRB 101 training in person. Unless in-person training is requested, the IRB 101 training will be done completely online. Departments can request training for their group on particular topics if needed by contacting irb@ohsu.edu.
The Introduction to the IRB courses (Overview and Getting Started) offer a brief history of institutional review boards (IRB), tips for submitting your human subjects research for IRB review and working with Non-OHSU collaborators.
You will also learn basic information about the review process for IRB approval at OHSU:
- Documents and information commonly required for study submissions
- Navigating the eIRB system
- Overview of submissions- Initials, Modifications, Continuing Reviews, and Reportable New Information (RNI's)
This introductory course is strongly recommended for those new to human subject research or research staff new to OHSU but may also be a helpful refresher for those who have questions about the eIRB system or the OHSU IRB process.
The New Common Rule was implemented on January 21, 2019, with a few provisions scheduled for implementation in 2020. We are awaiting confirmation from the FDA that these changes will also apply to FDA-regulated research.
What researchers need to know:
- All new studies approved on or after 1/21/2019 will be subject to New Common Rule requirements.
- Research approved prior to 1/21/2019 is still subject to the Old Common Rule.
- These studies may be converted to the New Common Rule, which will be determined on a case-by-case basis, based on whether it can easily meet all the new requirements and will offer review process streamlining.
- Existing studies will NOT be automatically transitioned.
- The Consent Form templates posted in December 2018 are compliant with both the Old Common Rule and the New Common Rule, and should be used for:
- Any new study submissions.
- An entirely new consent form being added to an existing study currently under the Old Common Rule.
- Existing studies do NOT need to change their existing consent forms to the current revised Consent Form templates. However, an existing study cannot be transitioned to the New Common Rule (after 7/29/2018) unless it meets all the New Common Rule requirements including the new consent elements.
Note: Always visit the IRB Policies and Forms page Consent Form section for the latest version of any Consent Form templates.
The main changes under the New Common Rule:
- Consent Form (see the revised templates)
- Consent forms need to start with a "clear and concise summary" of "key information" for the subject.
- Additional new required statements (note: most of these are already in our template, but are now specifically required):
- Whether information or biospecimens will or will not be used for future research.
- Whether research results will be returned to subjects or not.
- Whether research will include whole genome sequencing.
- Whether biospecimens will be used for commercial profit.
- Consent forms will be required to be posted on a (yet-to-be-determined) federal website within 60 days after the last study visit.
- IRB review process
- New exempt categories for "benign behavioral interventions" in adults with consent.
- Revision to educational exempt category.
- Continuing review
- Expedited studies and all studies once they reach "clinical care follow-up" or "data analysis only" stages will no longer require continuing review.
- These studies will still have an annual "check-in" to determine if the study is still ongoing and have an annual compliance review (all staff are trained, have CoI disclosures, etc.).
Please contact Research Integrity with any questions regarding changes due to the New Common Rule.
Common questions
If you are new to OHSU or just new to human research at OHSU, specific onboarding steps must be completed before you can be listed as a study team member on a human subject study in the eIRB system. In addition to any institution or department required trainings you must complete, federal regulations require the study staff of human research protocols to complete certain research training, depending on the type of study, and to complete an annual Conflict of Interest (CoI) in Research Disclosure form.
Onboarding instructions for the eIRB will vary depending on what category you belong to:
- New Human Researchers:
- OHSU employees
- Non-OHSU Researcher Collaborators:
- OHSU affiliated non-employees (e.g., OHSU students, volunteers, etc.)
- External collaborators (non-affiliates) intending to complete OHSU training or CoI disclosures
- Onboarding external collaborator FAQ (#8 under PI and Study Team Management):
- External collaborators that already have up-to-date research training and CoI completed through their home institution
If your project involves human subjects or their data, the best practice is to check with the IRB to confirm if IRB oversight is required. If you are not sure whether your project requires IRB oversight (such as for Quality Improvement or Quality Assurance projects), you can submit a Request for Determination Form. You would still create a new study in the eIRB system, but instead of uploading a Protocol, you would upload the Request for Determination Form. The IRB would review the form to determine if your project requires IRB oversight, which would then require a full study submission, or if your project can proceed as described without oversight.
When IRB oversight is required, your eIRB submission must be reviewed and approved before you start your project. For more information about what the IRB must review and how the review process works, see the Investigator Manual on the IRB Policies and Forms page.
#section-200546All submissions to the IRB are handled electronically in the eIRB system. This includes Requests for Determination in which you are trying to find out if your project requires IRB oversight.
You can find all of the forms needed for submission, as well as policies, procedures, guidance and instructions, on our IRB Policies and Forms page.
If you are new to the IRB review process, the Investigator Manual and the General Researcher Resources section of the IRB Policies and Forms page is a good place to start. The Initial Submission Guide (Quick Guide) provides guidance on what to include in an initial submission, and the Initial Submission Guide (Step-by-Step Guide) details how to fill out your submission in the eIRB system.
The Initial Submission Guide (Step-by-Step Guide) and the Modifications and Continuing Reviews (Step-by-Step Guide) explain how to create and fill out Initial submissions and follow-on submissions, like Modifications and Continuing Reviews.
Our Frequently Asked Questions (FAQ) page also contains information on how to perform common actions in the eIRB system.
We also offer the Introduction to the IRB class which includes a demonstration of the basic functionality of the eIRB system. This class is offered every third Thursday of the month. See the Education and Resources page for information on upcoming sessions.
HIPAA and research
There are various types of HIPAA protections that apply to the use and disclosure of Protected Health Information (PHI) for research purposes. See the Information Privacy and Security page about HIPAA or more specifically HIPAA and Research for details.
Just like improper disclosures of PHI for any other reason, improper disclosure of PHI in the context of a research study must be reported immediately to the Information Privacy and Security Office.
HIPAA gives all OHSU patients and research subjects the right to request an accounting of certain disclosures of their Protected Health Information (PHI). In order to provide an Accounting of Disclosures if requested, OHSU must track those disclosures. For information about the Accounting of Disclosure System, see the Accounting of Disclosure System (ADS) instructions.
Tracking Required
Disclosures made to another institution under the following:
- Waiver of HIPAA Authorization
- Decedents Representation
- Business Associate Agreement
Tracking NOT Required
- Disclosures of a Limited Data Set under a Data Use Agreement
- Disclosures authorized by a subject per a signed Consent and Authorization form
- Disclosures of health information that does not contain any of the 18 HIPAA identifiers
- Use of PHI within OHSU under a Waiver, Prep to Research, or Decedents Representation
Check out Accounting of Disclosures and Accounting of Disclosures Guidance and for more information.
Disclosures of PHI are tracked at OHSU in the electronic Accounting of Disclosures System (ADS) . Step-by-step instructions are available to explain how to access and use the ADS, as well as the Accounting of Disclosures Guidance document.
For studies that plan to enroll 50 or more subjects, make a single entry in the ADS at the beginning of the study. For studies with less than 50 subjects, enter each individual subject into the ADS as they are enrolled.
Effective January 1, 2015, the IRB is adding a new question to its HIPAA - Waiver or Alteration of HIPAA Authorization and HIPAA - Decedents Representation Form forms. If you submit one of these forms and indicate that you are disclosing PHI outside of OHSU, you will also need to indicate your plan for compliance with Accounting of Disclosures requirements by selecting one of the following options:
- "This study will enroll 50 or more subjects. The study was entered into the Accounting of Disclosures System on (date):________"
OR
- "This study will enroll fewer than 50 subjects. The person responsible for entering each subject into the Accounting of Disclosures System is:_________"
You are not required to enter past or ongoing studies/subjects into the ADS, but you must use the ADS for Waivers of Authorization or Decedents Representations submitted to the IRB on or after January 1, 2015.