Diagnostic Services
Offering doctors and patients a wide range of diagnostic services
We have a full service ocular pathology laboratory, medical photography and an ocular immunology laboratory where testing is carried out for autoimmune diseases and cancer-associated diseases that affect the eye.
Ophthalmic imaging
The Casey Eye Institute's ophthalmic imaging service uses photography and ultrasound images to provide vital information for diagnosing and treating eye conditions and diseases.
Ocular Immunology Lab
The Ocular Immunology Laboratory at Casey offers several anti-retinal autoantibody tests that can provide vital information on a range of eye conditions, including cancer-associated retinopathy (CAR), melanoma-associated retinopathy (MAR), autoimmune retinopathy and age-related macular degeneration (AMD).
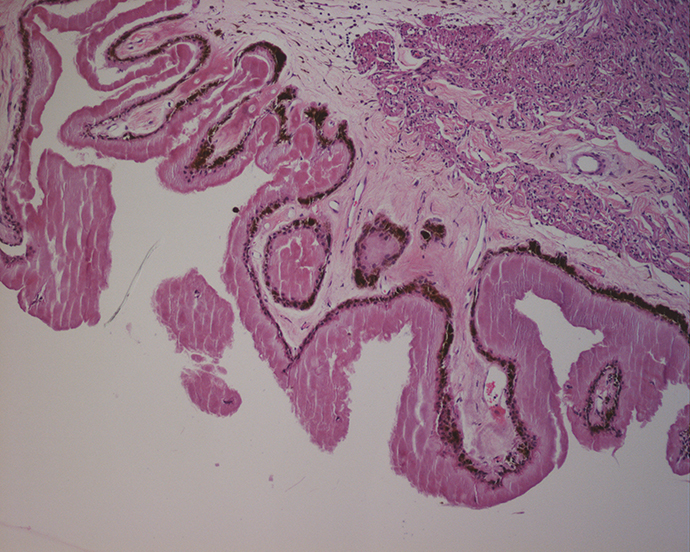
Ocular pathology
The Leonard Christensen Eye Pathology Laboratory at Casey is the only full-service eye pathology laboratory in the Pacific Northwest certified through federal regulations outlined by the Clinical Laboratory Improvement Amendments of 1988. Our laboratory staff provides a range of histopathological analyses of surgical and experimental eye tissues.