Embryo and Sperm Cryopreservation Background
Background
Exponential growth of mutant mouse lines and the number of animals required for definitive studies has led to exponential growth in animal costs and challenges for colony management. Cryopreservation of mouse germplasm(embryos and sperm) is an efficient and cost effective solution for managing colony size. In addition, this service permits archiving of transgenic models not currently needed but potential invaluable to future studies and provides protection against spontaneous mutations occurring in subsequent generations maintained in the live colony. Cryopreservation of mouse strains has traditionally relied on freezing embryos at the 2-cell or 8-cell stage, mainly because live mice can easily be reconstituted by embryo transfer (Whittingham et al., 1972).
An alternative to embryonic cryopreservation is sperm cryopreservation followed by in vitro fertilization (IVF) (Critser, Mobraaten, 2000). However, reconstituting live mice by IVF from frozen-thawed sperm is inefficient with some strains, notably with the C57BL/6 strain, the most common background on which induced mutants are bred (Sztein et al. 2000). The main problem of C57BL/6 sperm is that frozen-thawed spermatozoa are inactive, due to a requirement of an incubation within the female tract for the activation. Moreover, the spermatozoa of frozen sperm samples have no or reduced motility often, therefore reconstituting of a mouse strain can only be achieved by an intracytoplasmic sperm injection (ICSI) into unfertilized oocytes (Wakayama, Yanagimachi, 1998;Li et al., 2009).
The most effective method of cryopreservation ofa mouse line is theSpeed Cryo approach (Byers et al., 2006). The method consists of superovulation of virgin females, following isolation of unfertilized oocytes and subsequent IVF using freshly collected sperm from males of the mutant strain. Following fertilization and culture, 2 or 8 cell embryos can be frozen using standard method. Ideally only 3-4 males are required to prepare and freeze 250-350 embryos. However, the efficiency of Speed Cryo depends on the effectiveness of IVF, strain background and the fertility of the individual sperm donors (Vergara et al., 1997;Byers, et al., 2006).

Cryo-preservation accessories used in our laboratory. (A) Eight cell embryos prepared for cryopreservation. (B) Plasticaccessories listing from top to bottom: concave cryogenic vials used for freezing ES cells, a conical cryogenic vial used for freezing of embryos/sperm/oocytes, cryogenic straws used for freezing of embryos/sperm/oocytes. (C) FTS controlled Freezer for embryo cryopreservation. (D, E) Taylor-Wharton extended time liquid nitrogen refrigerator and cryostorage system used for long term storage of germplasm.
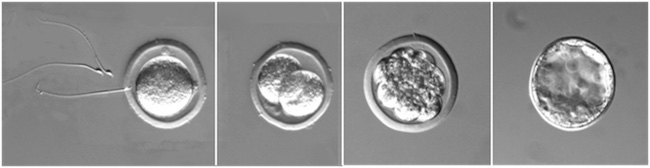
Development of mouse embryo after in vitro fertilization. From left to right: IVF process, 2-cell stage embryo, 8- cell morula, and blastocyst.