Logan Lab
Lab overview
In the mammalian brain, glial cells far outnumber neurons. Despite their abundance, we know surprisingly little about how glial cells communicate with neurons to regulate proper development and function of the nervous system. One striking and conserved attribute of glia is their ability to sense and respond to changes in neuronal health. Glia react quickly and robustly to neuronal death or damage after mechanical injury or stroke by infiltrating trauma sites and clearing damaged neurons through phagocytic engulfment. These glial immune responses are also invoked as neurodegenerative disorders, including Alzheimer’s Disease (AD) and Parkinson’s Disease (PD), progress over years. In the diseased brain, neuronal activity (and neuronal circuitry strength) slowly becomes compromised, which ultimately causes the gradual demise of neurons in the aging brain. Notably, in many situations, glial immune responses walk a fine line between being helpful and destructive; although important for minimizing post-traumatic damage, glia may also exacerbate the progression of some long-term neurodegenerative disorders.
Our goal is to elucidate the molecular and cellular pathways that govern neuronal-glial interactions in the healthy and diseased brain. We use the genetically powerful fruit fly Drosophila melanogaster as in vivo system, combined with molecular, genetic, and imaging approaches, to explore how glia detect and respond to damaged, dying, and remodeling neurons.
Exciting questions we are tackling include: (1) What signals are produced by degenerating neurons? (2) What are the cellular pathways that control glial migration to trauma sites and the phagocytic activity of glial cells? (3)
Lab members

Mary A. Logan
Ph.D. University of Utah, 2005
Post-doctoral Fellow, University of Massachusetts Medical School, 2005–2010
Faculty profile
loganm@ohsu.edu
503-418-1568
Mary joined the Jungers Center for Neurosciences Research in the Neurology Department as an Assistant Professor in September 2010 and was promoted to Associate Professor in 2017. She is also a faculty member with the OHSU Neuroscience Graduate Program.
After working at NPS Pharmaceuticals, Inc. in Salt Lake City, UT for several years, Mary graduated with a Ph.D. in Neuroscience from the University of Utah where she studied early neuronal differentiation gene transcription networks with Dr. Monica Vetter. Her postdoctoral work with Marc Freeman at the University of Massachusetts Medical School (Worcester, MA) explored immunological activity of glial cells in the fruit fly Drosophila melanogaster, with a particular emphasis on elucidating the molecular pathways that are critical for glia to sense and respond to degenerating neurons in the adult brain.
Current lab members
Cole Brashaw
Cole received a B.S. in Neuroscience from Drake University in 2019. As an undergraduate, he worked with Brian Sanders, exploring neurobiological, cardiovascular, and behavioral responses in several stress-induced rodent models. He also spent one summer interning with Donal Brennan at Systems Biology Ireland used a 3D cell culture to model ovarian cancer in an in vitro system. As a Neuroscience graduate student in the Logan lab, he now uses adult Drosophila to study the underlying molecular mechanisms that control how glial detect and respond to injury in the adult nervous system. Using a fly model of Alzheimer’s Disease, he is also investigating the importance of glial immune activity, including engulfment of toxic amyloid-beta (Aβ) aggregates, in disease pathology and progression.

Annie Griffin
Annie earned a B.S. in Biology from Portland State University in 2023. As an undergraduate, she worked on a herpetology project focused on characterizing the impacts of an invasive bullfrog species on a native frog population in the state of Oregon. She began working in the Logan lab part-time as an undergraduate researcher while a senior at PSU, and then joined the lab full-time as a Research Assistant after graduation. In the Logan lab, Annie performs essential tasks to keep the lab running smoothly (fly maintenance, husbandry, genetics, etc.) and is also contributing to ongoing experimental studies to explore glial immune responses following neuronal injury in adult Drosophila.
Former lab members

Derek Musashe
B.S., Furman University, South Carolina
Ph.D., Neuroscience, Oregon Health and Science University
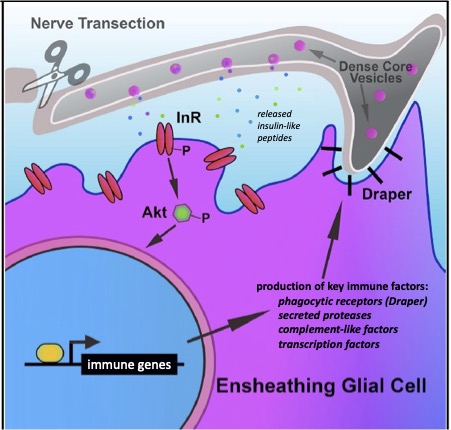
Derek completed his B.S. in Neuroscience from Furman University, where he studied neuronal circuits involved in short-term memory formation in Bill Blaker’s lab. As a Neuroscience Ph.D. student in the Logan lab, Derek defined a unique role for Insulin-like Signaling (ILS) as a novel acute injury communication relay, whereby severed axons release insulin-like peptides to active the ILS pathway in glia to trigger critical immune responses, including immune gene activation and glial phagocytic activity.
Derek is now Associate Director, Medical Science Liason at Amicus Therapeutics.

Maria Purice
B.S., University of Oregon
Ph.D., Neuroscience, Oregon Health and Science University
Maria earned a B.S. in Biology from the Robert D. Clark Honors College at the University of Oregon. During her undergraduate career, she worked with Chris Doe, investigating neuronal gene expression patterns of late-stage Drosophila embryos. She continued this project as a Summer Undergraduate Scholar at Janelia Farms Research Campus. Her undergraduate work also included the study of central pattern generators and how they can be visualized and manipulated during Drosophila larval locomotion. As a Neuroscience graduate student in the Logan lab, Maria investigated the effects of aging on glial immune gene expression and function in adult Drosophila, and also performed a large-scale RNAseq screen to identify new players in glial responses to degenerating neurons.
Maria is now a post-doctoral scholar in the laboratory of Aakanksha Singhvi at the Fred Hutch Cancer Center (Washington), where she is exploring the role of glia in circuit formation, maintenance, and aging using the nematode Caenorhabditis elegans as model system.

Lilly Winfree
B.S., University of Rochester, NY
Ph.D., Neuroscience, Oregon Health and Science University
Lilly received her B.S. in Neuroscience from the University of Rochester. As an undergraduate, she worked in Steve Goldman's lab studying oligodendrocyte precursor cells. She became interested in neuroscience in college during an introductory psychology class when she realized how integral proper brain function is to human behavior and health. Over time, she developed a strong interest in studying glial cell function because these cells are so poorly understood. In the Logan Lab, she used Drosophila as an in vivo system to investigate mechanisms of glial-neuronal interactions.
Lilly is currently working as a Senior Product Manager at data.world.

Petra Richer
B.S., University of the South, TN
Ph.D., Neuroscience, Oregon Health and Science University
Petra became interested in neurodevelopmental and neurodegenerative disorders while earning her Bachelor’s degree in Biology from the University of the South. She then joined a science exchange program and worked with Thomas Fernandez at Yale University where she studied the genetic underpinnings of Tourette’s Syndrome using exome sequencing analysis. Petra then joined the Logan as a Neuroscience graduate student and investigated how degenerating axons signal to glial cells to elicit immune responses. Using a variety of injury models, her work has provided new insight into the spatial and temporal regulation of glial immune gene expression at the transcriptional and translational levels.
Petra is currently the Science Communication and Medical Manager at Flywheel Partners.

E. Jolanda Muenzel
M.D., Medical University of Vienna, Austria
Ph.D., University of Edinburgh, United Kingdom
As a doctoral student in the laboratory of Anna Williams, Jolanda studied remyelination in zebrafish, a model organism known for its tremendous regenerative capacity. Her work explored molecular mechanisms of oligodendrocyte precursor recruitment and remyelination in adult zebrafish after injury, revealing exciting differences in the remyelination potential of young versus aged zebrafish, as well as putative signaling pathways driving remyelination. As a postdoctoral fellow in the Logan lab, Jolanda investigated the role of glial phagocytic activity in remodeling fine neural circuits and synaptic connections in the adult brain.
She is currently an Executive Medical Director at Eli Lilly and Company.

Arpita Ray
Ph.D., University of Alabama
As a doctoral student, Arpita worked with Guy Caldwell and Kim Caldwell at the University of Alabama, using C. elegans as a model organism to understand the fundamental mechanisms and genetic pathways involved in Parkinson's disease. She discovered the underlying mechanism of a bacterial secondary metabolite that causes human neuronal cell death as a result of mitochondrial dysfunction and oxidative stress. She also characterized a novel protein, named RtcB, in RNA metabolism and neuroprotection. To expand her knowledge in neurodegenerative disease mechanisms, she joined the Logan lab for her postdoctoral training. She used Drosophila to define the important contributions of glial immune function in attenuating the progression of Alzheimer's disease (AD). Specifically, she used a well-established fly model of AD, in which pathogenic human amyloid-beta (Aβ) is overexpressed in fly neurons. Using this system, she found that as harmful Aβ aggregates form in the adult brain, glial cells respond by upregulating innate immune and phagocytic receptors to clear these toxic proteins and slow disease progression in AD model flies.
Arpita now works as a Principal Scientist at Genentech (San Francisco, CA).

Matthew Boisvert
B.S., Bard College, NY
Ph.D., Salk Institute for Biological Sciences, CA
Matt became intrigued with how the brain works on a molecular level as an Undergraduate Distinguished Science Scholar at Bard College in New York. For his doctoral studies, he then joined Nicola Allen’s lab at the Salk Institute in San Diego and investigated how mammalian astrocytes change with age in various brain regions. He found that aging astrocytes share features with young astrocytes in a mildly injured or diseased brain, including enhanced expression of inflammatory factors such as complement. These findings prompt interesting questions regarding the role of complement factors during aging. Matt then joined the Logan lab as a postdoctoral fellow to leverage the genetic power of Drosophila and explore how complement-like factors contribute to glial immune activity in the adult brain during normal aging, as well as in response to nerve injury.
Matt is currently an Application Scientist at Araceli Biosciences.
Research
Dissecting complex glial responses to nerve injury
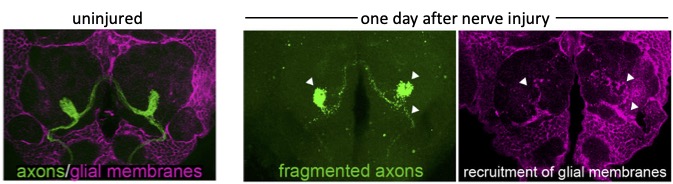
We can acutely trigger axon degeneration in adult flies by mechanically severing GFP-labeled antennal nerves of the olfactory system, which project into the antennal lobes of the central brain. Within hours, glial membranes accumulate on degenerating axons (Figure 1, arrowheads) and clear axonal debris from the brain. Notably, the adult fly brain contains a variety of glial subtypes that are morphologically, molecularly, and functionally similar to mammalian glia. In the context of the olfactory system, ensheathing glia are assigned to respond to degenerating axons after injury. These cells send extensive projections throughout the antennal lobes to enwrap and provide support to axons and synaptic-rich regions (Figure 2).
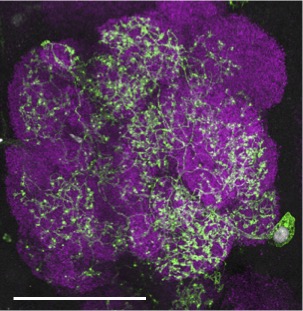
Our research, which includes RNA-seq analysis, live imaging of injury sites, and high-resolution imaging of glial cells, is revealing an increasingly complex model in which severed axons release injury cues to trigger expression of critical immune genes in ensheathing glial cells, allowing them to make their way to sites of injury and efficiently clear degenerating neuronal material (Figure 3).
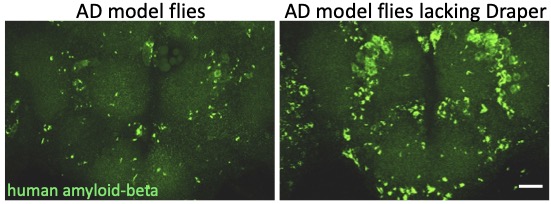
The role of glia in Alzheimer’s Disease
One hallmark feature of Alzheimer’s Disease (AD) is the progressive aggregation of toxic amyloid-beta (Ab) peptides in the brain. Studies of Ab pathology have been largely neuron-centric, but it is becoming clear that genetic risk factors, age-related changes, and dysfunctional immune activity all inform glial contributions to the onset and progression of AD, as well as other neurodegenerative disorders. To better understand how innate glial immunity is coupled to Ab aggregate formation and perdurance in the brain, we use a well-characterized Drosophila model of AD in which human Ab peptides form classic aggregates, eventually promoting behavioral defects, neurodegeneration, and reduced lifespan of adult flies. We find that many immune factors employed by glia as they respond to acute nerve injury are also activated in AD model flies. The glial phagocytic receptor Draper (fly ortholog of mammalian MEGF10) is upregulated in response to Ab aggregates in the brain and plays a key role in slowing the accumulation of this toxic protein (Figure 4). Leveraging new immune factors identified in our nerve injury screen, we continue to explore the role of candidate innate immunity genes that may influence glial cell recognition, internalization, and metabolism of neurotoxic peptides in AD model animals. (e.g. matrix metalloproteinases, Ninjurin, complement-like factors).
Publications
Richer P, Speese SD, Logan MA. FLARIM v2.0, an improved method to quantify transcript-ribosome interactions in vivo in the adult Drosophila brain. BioRxiv
Winkler B, Funke D, Benmimoun B, Spéder P, Rey S, Logan MA, Klämbt C. Brain inflammation triggers macrophage invasion across the blood-brain barrier in Drosophila during pupal stages. Sci Adv. 2021 Oct 29;7(44).
Raiders S, Han T, Scott-Hewitt N, Kucenas S, Lew D, Logan MA, Singhvi A.J. Engulfed by Glia: Glial Pruning in Development, Function, and Injury across Species. Neurosci. 2021 Feb 3;41(5):823-833.
Logan MA, Speese SD. In Vivo Analysis of Glial Immune Responses to Axon Degeneration in Drosophila melanogaster. Methods Mol Biol. 2020 2143:321-338.
Logan MA. Glial contributions to neuronal health and disease: new insights from Drosophila. Curr Opin Neurobiol. 2017 Dec;47:162-167.
Ray A, Speese SD, Logan MA. Glial Draper rescues Abeta toxicity in a Drosophila model of Alzheimer’s disease. Journal of Neuroscience. 2017, Nov 6. pii: 0862-17.
Purice MD, Ray A, Münzel EJ, Pope BJ, Park DJ, Speese SD, Logan MA. A novel Drosophila injury model reveals severed axons are cleared through a Draper/MMP-1 signaling cascade, eLife. 2017 Aug 21;6:e23611.
Lin L, Rodrigues FSLM, Kary C, Contet A, Logan MA, Baxter RHG, Wood W, and Baehrecke EH. Complement-Related Regulates Autophagy in Neighboring Cells. Cell. 2017 Jun 29;170(1):158-171.
Logan MA. Fragile phagocytes: FMRP positively regulates engulfment activity. J Cell Biol. 2017 Mar 6;216(3):531-533.
Lu TY, MacDonald JM, Neukomm LJ, Sheehan AE, Bradshaw R, Logan MA, Freeman MR. Axon degeneration induces glial responses through Draper-TRAF4-JNK signaling. Nature Communications. 2017 Feb 6;8:14355.
Winfree LM, Speese SD, Logan MA. Protein phosphatase 4 coordinates glial membrane recruitment and phagocytic clearance of degenerating axons in Drosophila. Cell Death and Disease. 2017 Feb 23;8(2):e2623.
Musashe DT, Purice MD, Speese SD, Doherty J, Logan MA. Insulin-like signaling promotes glial phagocytic clearance of degenerating axons through regulation of Draper. Cell Reports. 2016 Aug 16;16(7):1838-50.
Purice MD, Speese SD, Logan MA. Delayed glial clearance of degenerating axons in aged Drosophila is due to reduced PI3K/Draper activity. Nature Communications. 2016 Sept 20; 7, doi:10.1038.
Logan MA, Hackett R, Doherty J, Sheehan A, Speese SD, Freeman MR. Negative regulation of glial engulfment activity by Draper terminates glial responses to axon injury. Nat Neurosci. 2012 15, 722-730.
Osterloh JM, Yang J, Rooney T, Powell EH, Fox N, Sheehan A, Avery M, Hackett R, Logan MA, MacDonald J, Ziegenfuss JS, Adalbert R, Hou Y, Nathan C, Ding A, Brown, Jr. R, Coleman M, Zuchner S, Tessier-Lavigne M, Freeman MR dSarm/Sarm1 governs a novel injury-induced axon death pathway. Science. 2012 Jul 27;337(6093):481-4.
McPhee CK, Logan MA, Freeman MR, Baehrecke EH. Activation of autophagy during cell death requires the engulfment receptor Draper. Nature. 2010 465, 1093-1096.
Fuentes-Medel Y, Logan MA, Ashley J, Ataman B, Budnik V, Freeman MR. Glia and muscle sculpt neuromuscular arbors by engulfing destabilized synaptic boutons and shed presynaptic debris. PLoS Biol. 2009 7(8), e1000184.
Doherty J*, Logan MA*, Tasdemir OE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J Neurosci. 2009 29, 4768-81. *co-first authors
Logan MA, Freeman MR. The scoop on the fly brain: glial engulfment functions in Drosophila. Neuron Glia Biol. 2007 3, 63-74.