Emery Lab
Lab overview
Lab overview
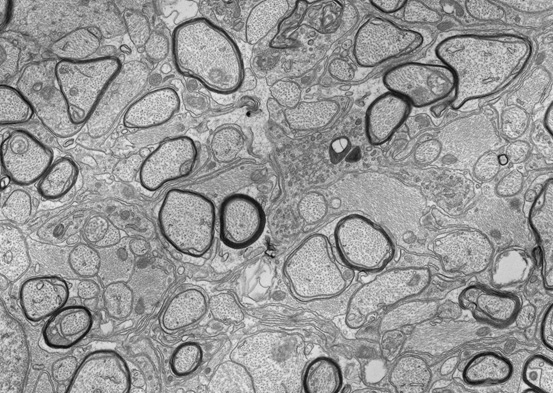
Glia make up around half of the cells in the human brain, supporting neurons and regulating almost every aspect of nervous system function. One of the most remarkable types of glia is the oligodendrocyte, which wraps multiple axons with spiraling layers of membrane to form myelin. This dramatically increases the speed and efficiency of action potential conduction along the myelinated axons, allowing for the complex sensory, motor and cognitive functions of the vertebrate nervous system. In addition, oligodendrocytes provide trophic and metabolic support to the axons. This means that the loss of oligodendrocytes and their myelin (as seen in diseases such as Multiple Sclerosis) both disrupts the conduction of nerve impulses and also leaves the neurons vulnerable to degeneration.
Our research seeks to uncover the molecular and cellular mechanisms controlling myelination in the CNS. In particular, we are interested in the genetic pathways that regulate the generation of oligodendrocytes and their subsequent myelination of axons. We also seek to understand how neurons and oligodendrocytes interact to ultimately determine which axons are myelinated. Finally, we aim to understand how loss of myelin impacts neuronal health and how to promote myelin repair in demyelinating disease (remyelination). Our lab uses a range of techniques including genetically modified mouse models, tissue culture, genome-wide sequencing and viral approaches to address these questions.
Lab members
Lab members

Ben Emery, Ph.D., emeryb@ohsu.edu
Principal Investigator
Warren Distinguished Professor in Neuroscience Research
Faculty profile
503-494-6329
Ben graduated from the University of Melbourne in 2005 before completing a postdoc at Stanford University with Professor Ben Barres in 2009. His postdoctoral research focused on defining gene expression across the different cell types of the brain and using this information to identify novel genes involved in myelination. Through this work he identified a gene (now known as Myrf) as a critical regulator of oligodendrocyte development and myelination.
Ben is the recipient of several awards, including the Australian Neuroscience Society A.W. Campbell Award, the Australian Institute of Policy and Science Victorian Tall Poppy Award and the American Anatomical Association Young Investigators Award in Morphological Sciences.
Ben is a faculty member of the Jungers Center for Neurosciences Research, the OHSU Neuroscience Graduate Program and the Graduate Program in Biomedical Sciences “D3” hub.

Greg Duncan, Ph.D.,duncangr@ohsu.edu
Postdoctoral Fellow
During his graduate studies, Greg was interested in understanding why myelin regeneration (remyelination) typically fails in myelin-destroying (demyelinating) diseases like multiple sclerosis (MS). He found the transcription factor MYRF to be essential for remyelination in rodents. He then went on to characterize MS lesions and discovered that MYRF is downregulated in non-remyelinating multiple sclerosis (MS) lesions and upregulated in lesions with successful remyelination. This implied that a failure to express MYRF in CNS-myelinating cells, the oligodendrocyte, underlies remyelination failure. Greg was funded by a Canadian MS Society studentship during his Ph.D.
Upon joining the Emery lab, he was interested in understanding the consequence of myelin loss on the neuron. To explore this question, he has successfully developed novel rodent models of genetic demyelination, which subsequently feature successful or impaired remyelination. He identified that in absence of remyelination, neurons were damaged. Greg went on to identify an intracellular stress cascade to be essential for neurodegeneration following remyelination failure. He has been awarded a National Multiple Sclerosis (NMSS) fellowship and Collins Medical Trust grant during his postdoctoral fellowship.
In the future, Greg wants to understand how the neurons respond and adapt to demyelination. His ultimate aim is to identify novel therapeutic strategies to protect neurons in demyelinating diseases like MS. Greg was recently awarded a NMSS career transition award to continue his studies in the Emery laboratory for two more years. This award provides an additional three years of support as a faculty member.

Hannah Collins,collinha@ohsu.edu
Graduate Student, Neuroscience Graduate Program
Hannah received her B.S. in Cellular Molecular Biology with a minor in psychology from Humboldt State University, on the beautiful northern coast of California, in 2015. There she studied the role of asymmetric cell division of neural stem cells in the initiation and progression of glioblastoma. After graduating she joined the lab of Dr. Claudia Petritsch at UCSF as a California Institute of Regenerative Medicine (CIRM) Bridges Fellow, continuing her work on asymmetric cell division, this time in oligodendrocyte progenitor cells (OPCs). In 2016 she joined the lab of Dr. Ben Barres at Stanford University as a research technician working on creating defined culture system for microglia.
Hannah joined the Neuroscience Graduate Program in 2018 and the labs of Dr. Ben Emery and Dr. Kelly Monk in 2019. With this co-mentorship she hopes to use the combined power of zebrafish and mouse models to understand the regulation and maintenance of central nervous system myelination.

Adam Coombs, coombsa@ohsu.edu
Graduate Student, Neuroscience Graduate Program
Adam began as a community college transfer student and received his B.S. in Biochemistry and Cell Biology from the University of California, San Diego in 2018. In addition to the gorgeous weather that San Diego has to offer — there is also an abundance of opportunities to collaborate with surrounding private institutions and industry partners. Adam was fortunate to have the opportunity to join the lab of Ardem Patapoutian at Scripps Research/HHMI. He began as an undergraduate intern before quickly transitioning into a lab technician, and after graduation was offered a full-time position as a research assistant.
During his time in the Patapoutian Lab, Adam had the opportunity to work on a number of exciting projects exploring the role of mechanosensitive ion channels in a variety of organisms — ranging from plants (Arabidopsis thaliana and the Venus flytrap) to highly translational projects in humans and mice.
Adam was part of the incoming 2020 cohort of the Neuroscience Graduate program at OHSU — and in 2021, joined the labs of Ben Emery and Kelly Monk. He hopes to build upon his experience working with mechanosensitive ion channels to explore the mechanobiology of myelinating glia.

Katie Emberley, emberley@ohsu.edu
Graduate Student, Neuroscience Graduate Program
Katie began her undergraduate studies at Northeastern University where she was fortunate to be a research assistant at Editas Medicine in Cambridge, MA. At Editas, her team developed a CRISPR/Cas9 gene therapy to treat sickle cell disease. She finished her undergraduate studies at the University of Vermont, receiving her B.S. in Neuroscience in 2021. There she studied dual tasking in persons with Multiple Sclerosis to improve protocols for fall risk assessment and exercise regimes. After graduating, she took the summer to explore the Northeast through hiking, mountain biking, and camping.
Katie joined the Neuroscience Graduate Program in 2021 and the lab of Dr. Ben Emery in 2022. She is interested in neuron-oligodendrocyte interactions. Specifically, studying the mechanisms of axon degeneration in a demyelinating context to elucidate therapeutic targets for axonal health preservation in diseases such as Multiple Sclerosis. In her free time, she takes to the surrounding mountains or coast with friends and family.

Jennifer Jenks, jenksj@ohsu.edu
Graduate Student, Neuroscience Graduate Program
Jennifer received her B.S. in Neuroscience from the University of Texas at Dallas in May 2021. Interested in studying neurodegenerative diseases, Jennifer joined the lab of Dr. Denise Park her freshman year and spent most of the subsequent three years investigating the role of learning and experience on cognitive function in older adults. In 2020, after learning about the complex role of glia in neuropathology, Jennifer transitioned to investigating oligodendrocyte-microglia interactions in demyelinating disease under Michele Binder and Dr. Trevor Kilpatrick at the Florey Institute of Neuroscience in Melbourne, Australia.
Following her love for glia biology to OHSU, Jennifer joined the Neuroscience Graduate Program in August 2021 and the Emery Lab a year later, where she continues to study oligodendrocytes in demyelinating disease, with a focus on injury-induced myelin plasticity.
Projects
Transcriptional control of oligodendrocyte development
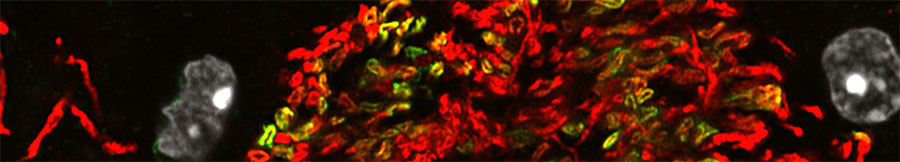
Oligodendrocytes are able to differentiate from their progenitor cells (and even form rudimentary myelin) in the absence of neurons, suggesting much of their development is genetically hard-wired. Our work identified Myelin Regulatory Factor (Myrf, previously known as C11Orf9, Gm98 and MRF) as a key component of this intrinsic differentiation process. The MYRF protein acts as a transcription factor, directly promoting the expression of several hundred other genes that underpin the myelination of axons.
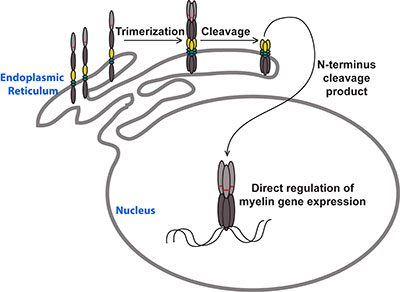
Although MYRF is a transcription factor, it is not a straightforward one. Our lab found that MYRF is a novel example of a membrane-associated transcription factor, being synthesized as an endoplasmic reticulum-bound transmembrane protein that needs to be cleaved before it can access the nucleus and bind DNA. Unexpectedly, this cleavage occurs via a mechanism that seems to have been borrowed from viruses, with a bacteriophage-related domain within the MYRF protein allowing it to trimerize and then self-cleave. This makes MYRF very different from other known membrane-associated transcription factors (such as Notch or the SREBPs), all of which require additional proteases for their activation. Our lab published these findings in 2013 back-to-back with a paper by Dr. Yungki Park from Edward Marcott’s laboratory, who made similar findings for the human MYRF protein. Our current work seeks to understand why the MYRF protein undergoes such a convoluted biogenesis and how recently described human mutations disrupt the protein’s function. We are also seeking to understand how MYRF fits into the broader program of CNS myelination, identifying its binding partners and gene targets in myelinating glia.
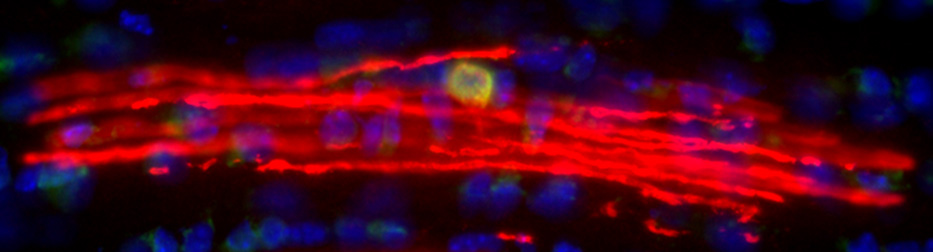
Oligodendrocyte-axon interactions during neuroplasticity
Historically, myelination appeared to be a relatively genetically-hardwired developmental process. It is now increasingly appreciated that not only can myelination occur throughout much of adult life, but also that myelination is responsive to neuronal activity. This raises the possibility that ongoing changes to myelin represent a form of neuroplasticity. Our recent work (with collaborators from University of Melbourne, Monash University, University of Queensland and University College London) has shown that neuronal activity promotes the generation of new oligodendrocytes in the adult mouse brain and that these new oligodendrocytes prefer to myelinate activated axons compared to their less active neighboring axons. Consistent with a role for myelination in neuroplasticity, genetically blocking new myelination (by deleting the Myrf gene in adult oligodendrocyte progenitors) disrupts normal motor learning. Our lab is currently using a range of in vivo techniques (viral CRISPR, DREADDs, TRAP-Seq) to better understand how neurons, oligodendrocytes and their progenitors interact in the adult brain during plasticity.
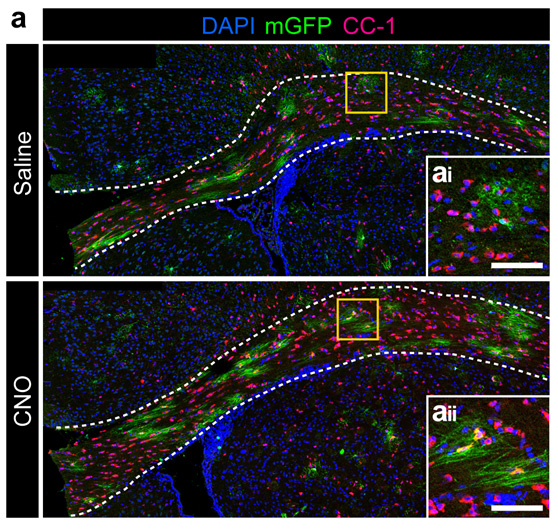
Oligodendrocyte-axon interactions in demyelinating disease
Myelin is destroyed in a number of human diseases, most notably Multiple Sclerosis (MS). Axonal loss is also a key feature in MS, most likely driving the ultimate clinical progression of the disease. The exact contributions of chronic demyelination and inflammation to axonal degeneration remain poorly understood, however. Our lab has generated novel genetic mouse models of chronic demyelination to better understand how neurons normally respond to loss of their myelin and how we might be able to promote neuronal resilience to chronic demyelination. We are also collaborating with other labs at OHSU to use our genetically modified mouse models as preclinical models to test novel drugs that promote myelin repair.
Publications
Publications
View full list of publications on PubMed
Duncan GJ, Ingram SD, Emberley K, Hill J, Cordano C, Ahmed A, McCane M, Jabassini N, Ananth K, Ferrara SJ, Stedelin B, Aicher SA, Scanlan T, Watkins TA, Mishra A, Nelson J, Green AJ, Emery B. Remyelination protects neurons from DLK-mediated neurodegeneration. BioRxiv [Preprint] bioRxiv 2023.09.30.560267; Available from: https://doi.org/10.1101/2023.09.30.560267
Ferrara SJ, Chaudhary P, DeBell MJ, Marracci G, Miller H, Calkins E, Pocius E, Napier BA, Emery B, Bourdette D, et al. 2022. TREM2 is thyroid hormone regulated making the TREM2 pathway druggable with ligands for thyroid hormone receptor. Cell Chem Biol 29: 239-248.e4.
Hay CM, Jackson S, Mitew S, Scott DJ, Koenning M, Bensen AL, Bujalka H, Kilpatrick TJ, Emery B. 2021. The oligodendrocyte-enriched orphan G protein-coupled receptor Gpr62 is dispensable for central nervous system myelination. Neural Dev 16: 6.
Carlie L. Cullen, Renee E. Pepper, Mackenzie T. Clutterbuck, Kimberley A. Pitman, Viola Oorschot, Loic Auderset, Alexander D. Tang, Georg Ramm, Ben Emery, Jennifer Rodger, Renaud B. Jolivet, Kaylene M. Young. 2021 “Periaxonal and nodal plasticities modulate action potential conduction in the adult mouse brain” Cell Reports 34(3):108641.
P. Chaudhary, G. H. Marracci, E. Calkins, E. Pocius, A. L. Bensen, T. S. Scanlan, B. Emery and D. N. Bourdette. 2021 Thyroid hormone and thyromimetics inhibit myelin and axonal degeneration and oligodendrocyte loss in EAE. Journal of Neuroimmunology 352:577468.
Wenxian Wang, Hyeyoung Cho, Dongkyeong Kim, Younjung Park, Ji Hwan Moon, Su Jeong, Lim Sung, Min Yoon, Michael McCane, Sue A. Aicher, Sangsoo Kim, Ben Emery, Jae W. Lee, Seunghee Lee, Yungki Park, Soo Kyung Lee. 2020 PRC2 Acts as a Critical Timer That Drives Oligodendrocyte Fate over Astrocyte Identity by Repressing the Notch Pathway. Cell Reports 32(11):108147.
Meredith D. Hartley, Tania Banerji, Ian J. Tagge, Lisa L. Kirkemo, Priya Chaudhary, Evan Calkins, Danielle Galipeau, Mitra D. Shokat, Margaret J. DeBell, Shelby Van Leuven, Hannah Miller, Gail Marracci, Edvinas Pocius, Tapasree Banerji, Skylar J. Ferrara, J. Matthew Meinig, Ben Emery, Dennis Bourdette, Thomas S. Scanlan. 2019 Myelin repair stimulated by CNS-selective thyroid hormone action. JCI insight 4(8):e126329.
Sarah J Garnai, Michelle L Brinkmeier, Ben Emery, Tomas S Aleman, Louise C Pyle, Biliana Veleva-Rotse, Robert A Sisk, Frank W Rozsa, Ayse Bilge Ozel, Jun Z Li, Sayoko E Moroi, Steven M Archer, Cheng-Mao Lin, Sarah Sheskey, Laurel Wiinikka-Buesser, James Eadie, Jill E Urquhart, Graeme C M Black, Mohammad I Othman, Michael Boehnke, Scot A Sullivan, Gregory L Skuta, Hemant S Pawar, Alexander E Katz, Laryssa A Huryn, Robert B Hufnagel, Genomic Ascertainment Cohort, Sally A Camper, Julia E Richards, Lev Prasov. 2019. Variants in myelin regulatory factor (MYRF) cause autosomal dominant and syndromic nanophthalmos in humans and retinal degeneration in mice. PLoS Genetics 15(5):e1008130.
Mitew, S., Gobius, I., Fenlon, L.R., McDougall, S.J., Hawkes, D., Xing, Y.L., Bujalka, H., Gundlach, A.L., Richards, L.J., Kilpatrick, T.J., Merson, T.D., Emery, B., 2018. Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nature Communications 1–16.
Duncan, G.J., Plemel, J.R., Assinck, P., Manesh, S.B., Muir, F.G.W., Hirata, R., Berson, M., Liu, J., Wegner, M., Emery, B., Moore, G.R.W., Tetzlaff, W., 2017. Myelin regulatory factor drives remyelination in multiple sclerosis. Acta Neuropathol 1–20.
McKenzie, I.A., Ohayon, D., Li, H., de Faria, J.P., Emery, B., Tohyama, K., Richardson, W.D., 2014. Motor skill learning requires active central myelination. Science 346, 318–322.
Bujalka, H., Koenning, M., Jackson, S., Perreau, V.M., Pope, B., Hay, C.M., Mitew, S., Hill, A.F., Lu, Q.R., Wegner, M., Srinivasan, R., Svaren, J., Willingham, M., Barres, B.A., Emery, B., 2013. MYRF Is a Membrane-Associated Transcription Factor That Autoproteolytically Cleaves to Directly Activate Myelin Genes. PLoS Biol 11, e1001625.
Emery, B., Dugas, J.C., 2013. Purification of Oligodendrocyte Lineage Cells from Mouse Cortices by Immunopanning. Cold Spring Harbor Protocols 2013, pdb.prot073973–pdb.prot073973.
Koenning, M., Jackson, S., Hay, C.M., Faux, C., Kilpatrick, T.J., Willingham, M., Emery, B., 2012. Myelin Gene Regulatory Factor Is Required for Maintenance of Myelin and Mature Oligodendrocyte Identity in the Adult CNS. Journal of Neuroscience 32, 12528–12542.
Emery, B., 2010. Regulation of oligodendrocyte differentiation and myelination. Science 330, 779–782.
Dugas, J.C., Cuellar, T.L., Scholze, A., Ason, B., Ibrahim, A., Emery, B., Zamanian, J.L., Foo, L.C., McManus, M.T., Barres, B.A., 2010. Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron 65, 597–611.
Emery, B., Agalliu, D., Cahoy, J.D., Watkins, T.A., Dugas, J.C., Mulinyawe, S.B., Ibrahim, A., Ligon, K.L., Rowitch, D.H., Barres, B.A., 2009. Myelin gene regulatory factor is a critical transcriptional regulator required for CNS myelination. Cell 138, 172–185.
Cahoy, J.D., Emery, B., Kaushal, A., Foo, L.C., Zamanian, J.L., Christopherson, K.S., Xing, Y., Lubischer, J.L., Krieg, P.A., Krupenko, S.A., Thompson, W.J., Barres, B.A., 2008. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. Journal of Neuroscience 344, 1252304–1252304.
Lab alumni
Lab alumni

Samantha Ingram
Research Assistant
Samantha graduated from Virginia Tech in 2021 with a B.S in Experimental Neuroscience and Psychology. At Virginia Tech, she worked in the laboratory of Dr. Mike Bowers, studying the neurobiology of language and communication disorders. Upon graduating Virginia Tech, Samantha moved from Virginia to Oregon and joined the Emery Lab as a Research Assistant. In 2023, Samantha left to attend graduate school to study the mechanisms of mental disorders, such as schizophrenia, to develop new therapies.