Projects
Alejandro Aballay's lab has a broad research program encompassing genetics, functional genomics and neurobiological approaches to study mechanisms involved in the control of immune responses against microbial pathogens. Immune activation needs to be fine-tuned since deficient or excessive inflammation can lead to cancer or other conditions such as Crohn’s disease, rheumatoid arthritis, atherosclerosis, diabetes and Alzheimer’s disease.
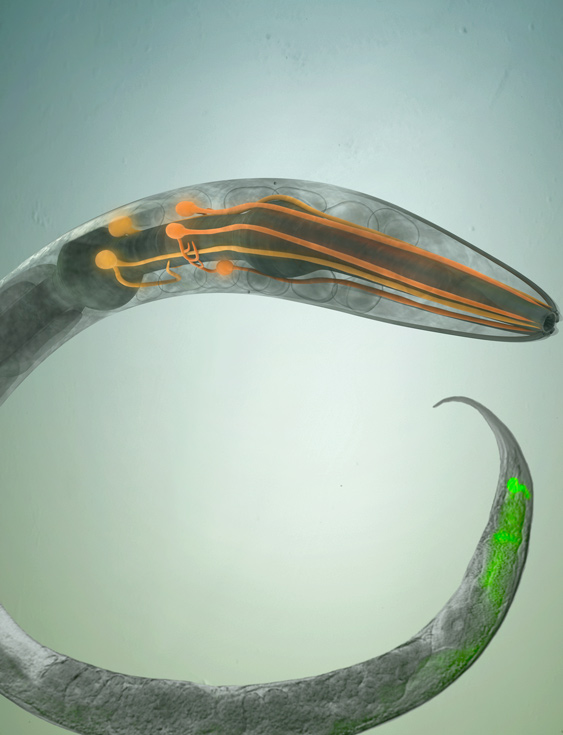
Recent studies from Aballay Lab indicate that different immune and cellular homeostatic mechanisms are regulated at the organismal level by the nervous system. The lab's research has demonstrated that specific neurons suppress innate immunity in the intestinal cells of the nematode Caenorhabditis elegans, in part by down-modulating a mitogen-activated protein kinase signaling pathway similar to the mammalian p38 MAPK pathway that is highly conserved across metazoans. The lab found that G-protein coupled receptors (GPCRs) participate in neural circuits that control a conserved p38/PMK-1 MAPK immune pathway and non-canonical and canonical XBP-1 unfolded protein response pathways that are expressed in non-neuronal tissues and that are necessary to alleviate the increased demand on protein folding during immune activation.
Alejandro Aballay's lab studies the following:
- Neuronal circuits involved in the control of immune homeostasis.
- Receptors involved in pathogen recognition.
- Signaling molecules involved in the control of immune homeostasis.
- Neural responses to infections such as pathogen-induced neurodegeneration.
- Molecular pathways involved in the control of recovery from bacterial infections.
Studies are based on the general hypothesis that immune pathways are regulated at the cell-autonomous level and, by the nervous system, at the cell non-autonomous level. The lab takes advantage of the simple and well-studied nervous and immune systems of C. elegans to develop a whole-animal high-throughput system for chemical and genetic screens for novel immunomodulators. They study the extent to which the basic cellular mechanisms identified in analyzing the role of selected genes and chemical compounds in mammalian stems.
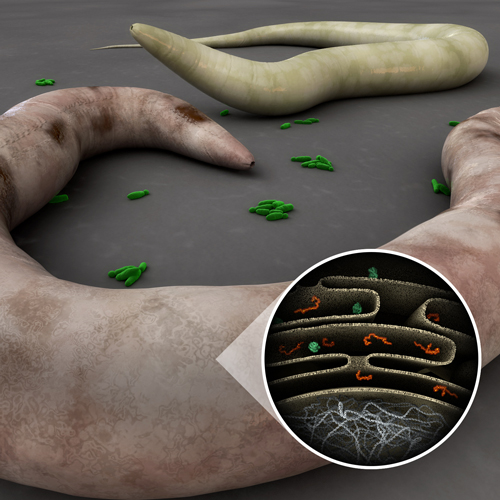
The complex and tractable C. elegans system is excellent to study all aspects of the molecular basis of host-pathogen interactions. From the perspective of the pathogen, the experimental advantage of using C. elegans as a host is that thousands of bacterial clones from a mutagenized library can be individually screened for avirulent mutants on separate Petri plates seeded with C. elegans. The advantages of using C. elegans to study host responses to pathogen attack are the extensive genetic and genomic resources available and the relative ease of identifying C. elegans mutants that exhibit altered susceptibility to pathogen attack.
Alejandro Aballay's lab uses the Caenorhabditis elegans system as a fast track to identify and initially characterize both host and pathogen genes involved in the pathogenic relationship. They study the role of these genes in a variety of mammalian systems. The lab uses a number of human pathogens including Salmonella enterica, Yersinia pestis, Pseudomonas aeruginosa, Staphylococcus aureus and Cryptococcus neoformans. Aballay Lab studies involve diverse disciplines including microbiology, genetics, immunology, and neurobiology.
Related information
Virulence Profile on Alejandro Aballay, Ph.D.
Virulence 4:7, 1–3; October 1, 2013; © 2013 Landes Bioscience
At the intersection of the nervous system and innate immunity.