Projects
Defining the mechanisms by which mycobacteria respond to and resist the antimicrobial repertoire of the macrophage
The ability to survive and multiply within the host macrophage is key to its pathogenesis. In resting macrophages, the bacterium arrests phagosome maturation and replicates in a compartment that resembles early endosomes. Activated macrophages promote clearance of mycobacteria through both non-oxidative and oxidative mechanisms: In activated macrophages there is increased fusion of the mycobacteria-containing vacuole with the lysosome where Ubiquitin-derived peptides (Ub-peptides) contribute to the bactericidal activity of the lysosome. Activation also triggers the production of reactive oxygen and nitrogen intermediates (ROI and RNI). Our work indicates that Ub-peptides act by physically interacting with mycobacterial membranes. This interaction disrupts the bacterial proton motive force and results in bacterial death (Purdy et al., 2009; Foss et al., 2012).
Understanding the role of MmpL proteins in the biosynthesis of the Mtb cell wall
The cell wall plays a crucial role in the Mtb host pathogen interface on several levels. The mycobacterial cell wall confers intrinsic resistance to external stresses including the host immune response and antibiotics. Mycobacterial cell wall lipids also contribute to biofilm formation. Genetic and biochemical studies have made great strides in the characterization of the mycobacterial cell wall. However, gaps remain in our knowledge: We have not identified all of the cell wall lipids, defined their biochemical pathways, or ascertained their importance in conditions mimicking those encountered during infection of the host. Recent work demonstrates that the Mycobacterial membrane protein large (MmpL) proteins transport lipids to the mycobacterial cell wall. The mechanisms underlying MmpL transporter function and their respective substrates are not fully elucidated. MmpL3 is essential and MmpL4, MmpL5, MmpL7, MmpL8 and MmpL11 contribute to Mtb virulence. Our work demonstrated MmpL11 transports the mycolic acid-containing lipids monomeromycolyl diacylglycerol (MMDAG) and wax ester mycolate (WE) to the M. smegmatis cell wall (Pacheco et al., 2013). Biofilm formation by the M. smegmatis mmpL11 mutant was impaired, but was complemented by expression of either M. smegmatis MmpL11 or MmpL11TB, emphasizing that MmpL11 has a conserved function in mycobacteria. We are continuing to investigate how the conserved mycolic-acid containing lipid transporters MmpL3 and MmpL11 function.
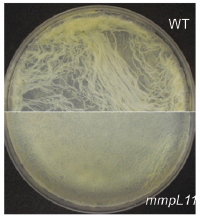
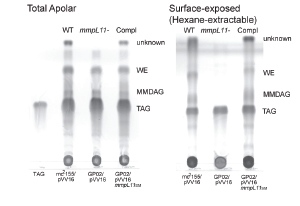
In collaboration with others we are developing and characterizing the mechanism of action of novel therapeutics against Mtb. Combined, this work will further define the pathogenic nature of Mtb, expand our knowledge of mycobacterial intrinsic resistance, and identify targets and new strategies for future drug therapy.