Projects
Our lab is interested in understanding how naïve progenitor cells segregate to give rise to diverse cell types that eventually form an organ. The question of organ formation is even more profound and challenging when applied to a vertebrate nervous system, where hundreds of cell types exist. We address this question in the context of cranial placodes, placode-derived sensory ganglia and zebrafish lateral line system. Development of placode-derived sensory components of the peripheral nervous system are essential for the formation of cranial sensory systems such as somatosensation and taste. The improper development of the cranial sensory system has been implicated in many human disorders, including chronic obstructive pulmonary disease, migraines, bladder overactivity, erectile dysfunction, heart failure, arrhythmia and others. Thus, uncovering genes that specify cranial placodes and ganglia should provide better understanding for the mechanisms underlying these.
The mechanosensory lateral line system of aquatic vertebrates is used to detect displacement of water and controls various types of swimming behavior. The lateral line provides an excellent system for studying basic biological processes, such as collective cell migration and specification, organ morphogenesis and patterning in the genetically-tractable model system such as zebrafish.
Development of cranial placodes and ganglia
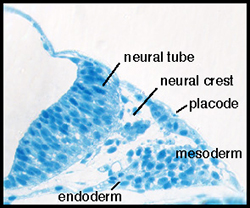
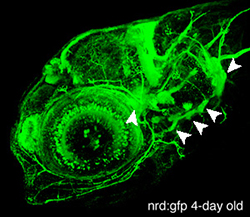
In vertebrates, placodes are transient epithelial thickenings (figure 1) within the nonneural ectoderm that give rise to sensory neurons of the cranial ganglia (figure 2, arrowheads) as well as the sensory structures of the nose and ear. The neurogenic placodes include the trigeminal placode that forms neurons of the trigeminal ganglia; the epibranchial (EB) placodes that generate the sensory neurons of the facial, glossopharyngeal and vagal ganglia; and lateral line placodes that give rise to the lateral line ganglia and mechanosensory neuromasts in aquatic vertebrates. EB neurons innervate internal organs to transmit information such as heart rate, blood pressure and visceral distension from the periphery to the CNS.
In our lab, we are interested in defining the cellular mechanisms of EB placode formation and uncovering roles of various signaling pathways during early placode development (reverse genetic approach).
In addition to a candidate gene approach, we are conducting a mutagenesis screen to find genes necessary for EB placode and ganglia development (forward genetic approach). We have already isolated a number of mutants defective in EB placode and ganglia development and efforts are underway to find mutated genes.
Development of lateral line system
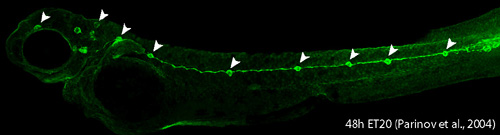
Lateral line system in aquatic vertebrates consists of mechanosensory organs called neuromasts (NM) (Figure 3, arrowheads) and lateral line nerves that innervate them. Each NM is a volcano-shaped structure with mechanosensory hair cells projecting microtubule-containing kinocilia and actin-based stereocilia through a central pore. Hair cells are surrounded by basally-located support cells. Since lateral line sensory cells are on the surface of the body, they are readily accessible for visualization and manipulation. The lateral line system is important for various behaviors such as feeding, schooling, obstacle avoidance and prey detection. The zebrafish lateral line is emerging as an excellent system to understand basic developmental events such as collective cell migration, proliferation and differentiation as well as mechanosensory hair cell development and regeneration in a relatively simple vertebrate system.