
OHSU Casey Eye Institute is a world leader in gene therapy, a groundbreaking technique for people facing blindness from hereditary eye disease.
Not only does Casey conduct the most clinical trials in gene therapy worldwide, it is one of a handful of eye centers to administer LuxturnaTM, the first FDA-approved gene therapy treatment for a rare childhood retinal disorder. In fact, its gene therapy team recently celebrated treating the 100th patient with gene therapy.
Casey’s prominence in ophthalmic genetics will rise even further thanks to a significant philanthropic investment from longtime donor Paul H. Casey to name the ophthalmic genetics division in the new Elks Children’s Eye Clinic.
“With our expanded space in the new building, the Paul H. Casey Ophthalmic Genetics Division will be well equipped to take on the upsurge in gene therapy trials and treatments, which are expected to quadruple within five years,” said Mark Pennesi M.D., Ph.D., division chief of ophthalmic genetics.
The new space will have all the unique elements required for a successful gene therapy program, including a life-size mobility maze to test vision after treatment and rooms specially designed to examine patients in complete darkness. There will also be more exam space and designated areas for the latest and most advanced diagnostic and imaging technology.
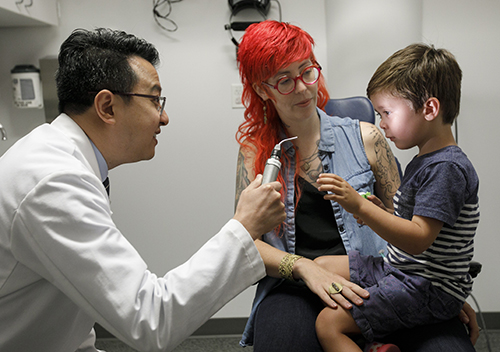
With gene supplementation therapy, non-working genes in the retina are supplemented with healthy copies to correct the underlying cause of the disease. The breakthrough approach has been shown to protect and in some cases, improve vision. The entire process involves specialized testing before and after the procedure, as well as the expertise of highly skilled surgeons able to perform the delicate operation.
Casey’s ability to offer this promising treatment is the culmination of decades of focused research, led by experts in ophthalmic genetics, retina, imaging technology and other specialties, said Pennesi, adding that philanthropic gifts both large and small have powered these accomplishments.
“Coupled with the support of a social worker and vision rehabilitation team, our program can offer the best possible care to patients and their families grappling with the life-altering diagnosis of a genetic eye disease.”
Related resources
Learn more about the Gene Therapy Center at OHSU Casey Eye Institute.
Article: High hopes for 4-year-old’s vision after gene therapy
Article: Innovations: Advancing Surgery