Sample Preparation
Important note about incompatible reagents:
The following reagents are among those that are incompatible with our mass spectrometers. If any of these are present in the samples being submitted please indicate the reagent and its concentration on the sample submission form. The reagent will have to be removed prior to running the sample.
Detergents (e.g. SDS, Triton, Tween)
Polyethylene glycol (PEG)
Many different polymers
HEPES (or other non-volatile buffer)
Phosphate salts
Other non-volatile salts
If you're not certain a reagent is compatible please list it anyway. This is a case where having too much information is better than having too little.
Sample preparation is one of the often overlooked steps in achieving high quality results in a proteomics experiment. Despite the enormous technical advances in mass spectrometric instrumentation and software, contaminated samples can easily overwhelm the most advanced mass spectrometer and extremely bad samples can actually render a mass spectrometer unusable for an extended period of time.
MAXIM – the cleaner your sample the better your data
Contaminated samples can cause delays in their own processing as well as with other samples passing through our core facility. What follows are some guidelines which can hopefully help your project avoid some of the more common problems. As for a couple of other sources of information, this short article titled The 11 Golden Rules of Working with Proteomic Samples does a good job of touching on several basic sample preparation concerns that can potentially derail a proteomics experiment. Also this 2014 paper discusses some of the concerns involved when interpreting biological significance from proteomics results.
The Proteomics Shared Resource does offer consulting for projects prior to sample submission. Consulting prior to submission of a grant is done free of charge, as we are reimbursed for our time from the University. Consultation prior to dropping off a set of samples is encouraged as well, especially with projects that have novel or unusual aspects to them. This time is included in the standard overhead for each project submission, and usually won't require additional fees.
Through consultation we may be able to help you identify ways you can alter your procedures so that you can better achieve your goals. For some experiments, especially those where you wish to look for differences in protein abundance, careful preparation is a must.
When it comes time for sample submission, the majority of the samples we accept consist of purified undigested protein mixtures (either in solution or lyophilized), or isolated proteins in a SDS-page gel. Depending on the physical state of the protein(s) at the time of sample submission we may have different concerns.
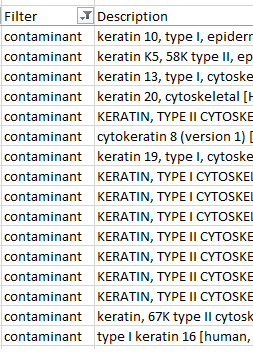
While not the massive challenge it used to be keratin contamination can still be an issue in proteomics. Time spent by the mass spectrometer analyzing peaks from keratin peptides is time not spent looking for proteins of interest. These tips will improve your chances of success.
- Analytical grade reagents and MilliQ water should always be used.
- Wear a lab coat and nitrile gloves at all times when handling your samples. After putting on gloves, rinse them under a stream of clean water before handling gels.
- Store consumables and reagents in covered containers, this includes: gels, all liquid and powdered reagents, pipette tips, etc.
- Do not re-use reagents at any time.
Take reasonable efforts to keep your workspace free of dust and lint. We typically clean our lab once every couple of months to keep this under control, and recommend doing the same.
As you may know from working in a laboratory setting over the years the majority of reagents come in one of two forms: clear liquids or white powders. Protein samples also fall into this category. What we likely receive upon sample submission is a rather plain looking sample, with no clue as to what is contained inside! Though we all hope there are proteins in the sample, the sad truth is there are other contaminants which can greatly hinder our ability to see those proteins we our instruments. Below is a list of chemicals and other contaminants that we know to be incompatible with our instruments.
- Significant particulate or insoluble material
- Radioactive or Biohazardous substances
- Salts: NaCl, KCl, Phosphates, and other non-volatile salts
- Detergents: SDS, NP-40, Triton, Tween, etc.
- Cyroprotectants: DMSO, Glycerol, etc.
- Cell debris: nucleic acids, mono- & polysaccharides, lipids, etc.
- PEG, and other polymers
If you are unsure about a chemical in your procedure please ask us about it. We will let you know if it has the potential to cause problems.
Samples with large amounts of insoluble material pose a threat mostly to our Liquid Chromatography (LC) system. Our chromatographic columns trap insoluble material upstream from them. Over time this material can build up, causing the pressure in the LC system to rise and distort the chromatography. In extreme cases the LC system can run over pressure, and halt analysis.
After this type of contamination is introduced, columns and LC lines may need to be replaced. We risk losing a couple of days or up to a week or more of instrument time, and may have to spend upward of $1,000 to replace unusable parts.
Samples containing radioactive or biohazardous substances are simply not accepted at PSR. We are not equipped to handle samples that are still considered to be biohazardous or radioactive. Please make sure any sample that is originally classified as biohazardous is made safe prior to submission.
As for radioactive samples we cannot afford to run them in our mass spectrometers as it violates the conditions of our service contracts.
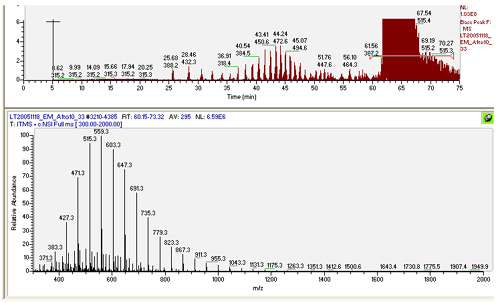
Samples contaminated with detergent are one of the worst things that can get put into a mass spectrometer. Unfortunately they are common in many procedures used to initially extract proteins. While detergent contamination can initially appear similar to salt contamination, the polymer problems associated with it are much more serious. Detergents and other similar polymers have several adverse affects:
- They form a m/z polymer series, similar to non-volatile salts, which competes for MS/MS scans
- They tend to ionize more readily than peptides sucking up electrons and lowering the signal from the peptides
- They get deposited on the column and take an extremely long time to wash off. Some polymers can remain in the system for weeks, diminishing sensitivity and resisting efforts to remove it. Often detergent contamination will render a column unusable, and we will be forced to dispose of it.
- They can coat the innards of a mass spectrometer, meaning the instrument itself may need to be taken apart and cleaned thoroughly. If it is bad enough a service engineer from the mass spectrometer's company may need to respond to clean the instrument.
It is not unusual for a bad case of detergent contamination to bring a mass spectrometer down for a month or more; costing thousands of dollars in lost time and replacement parts.
It is relatively easy to remove detergents from samples if we know it is present. For simple samples this often means we'll run it into an SDS-page gel and use the gel to catch the proteins, and carry away the undesirables. For samples where this isn't an option chromatography on a SCX or Waters MCX column is usually sufficient to remove all but the worst cases of contamination, and often simply running the sample a few centimeters into an SDS-page gel will be sufficient to address most problems. These procedures can be done at PSR if we are made aware of the presence of detergent in your samples. Dialysis usually isn't sufficient to dilute out the detergent as it doesn't take much to cause a large problem.
Fortunately samples submitted to us in a gel are usually free of many of the contamination issues that plague liquid & lyophilized samples. The SDS-page gel is one of the most cost effective ways to remove low amounts of several contaminating compounds, so much so that PSR regularly runs samples into a gel as a quick and inexpensive way to clean up samples given to us in liquid or lyophilized form. That being said gel samples have their own sets of problems.
The biggest potential contaminate is keratin, though there are others. Contamination is such a problem that we will NOT accept samples which have been:
- run on a gel poured in the lab. We only accept samples run on pre-packaged, pre-cast gels.
- stained with chemical stains made up in the lab. Please use commercially available staining reagents.
- placed in containers used for western blots, or other similar experiments. Please keep all SDS-page containers separate.
If you would prefer, gels can be run using the equipment and stains present in our lab. Please contact a member of PSR if you'd like to inquire about using our lab equipment.