Stephen Back Pediatric Research Lab
Program in regeneration and repair of cerebral white matter injury
The Back Lab focuses on the development of novel strategies to promote regeneration and repair after injury to the developing or adult brain.We employ several complementary approaches that utilize human tissues, tissue culture systems and unique animal models. We employ a cutting-edge multidisciplinary team approach that integrates studies utilizing cellular and molecular biology, electrophysiology, physiology, pharmacology, neuropathology, quantitative morphometry/stereology, spinning disc and laser confocal microscopy and high field MRI. We are continually "pushing the envelope" to grapple with the challenges of studying injury in the developing and adult nervous system.
We are studying several human diseases where cerebral white matter injury (WMI) is accompanied by disturbances in myelination.These include disorders at several stages of life where WMI is prominent: cerebral palsy in survivors of premature birth, multiple sclerosis, and vascular dementias.
We have found that all of these disorders share similar mechanisms in terms of the disturbances in the response of glial cells that disrupt the normal progression of myelination. We are currently studying molecules within the extracellular matrix that block white matter repair via an arrest in maturation of the oligodendrocyte progenitors required for normal myelination.We seek to develop molecular strategies to promote the generation of myelin in chronic white matter lesions with the long-term goal to restore motor and cognitive function lost from WMI.
We capitalize on the robust resources of the OHSU neuroscience community- one of the largest in the U.S. The OHSU Brain Institute has more than 1,000 scientists and clinicians. It is one of the nation's leading centers for neuroscience, and it ranks in the top two percent for competitive grant support from the National Institutes of Health.
Current projects
- Cellular responses to chronic white matter injury in the developing brain
- Alzheimer's Disease and Age-Related Cognitive Decline
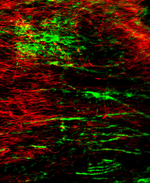
We have extensive experience with the collection, preservation and study of human brain tissue for neuropathology studies. We have a large collection of brain tissue collected at autopsy from premature and term infants that are linked to relevant clinical-pathological information. We have developed many immunohistochemical protocols that are optimized for the detection of human molecules in specific cell types within white and gray matter. On-going areas of study focus on mechanisms of chronic white matter injury related to alterations in key components of the extra-cellular matrix that are involved in myelination failure in the developing and adult CNS.
Back, S.A., N.L. Luo N.L., R.A. Mallinson, J.P. O'Malley, L.D. Wallen, B. Frei, J.D. Morrow, C.K. Petito, C.T. Roberts Jr., G.H. Murdoch, T.J. Montine. Human preterm cerebral white matter is selectively vulnerable to oxidative damage identified by F2-isoprostanes, Annals of Neurology 58:108-120, 2005.
Back, S.A., N.L. Luo, N.S. Borenstein, J.J. Volpe, H.C. Kinney. Arrested oligodendrocyte lineage progression during human cerebral white matter development: dissociation between the timing of progenitor differentiation and myelination. Journal of Neuropathology and Experimental Neurology 61(3):197-211 (2002).
Back, S.A., N.L. Luo, N.S. Borenstein, J.M. Levine, J.J. Volpe, H.C. Kinney. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. Journal of Neuroscience 22(4):1302-1312 (2001).
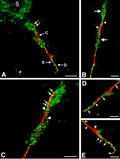
Maturation-dependent mechanisms of acute degeneration of the OLlineage from oxygen glucose deprivation (OGD)
We hypothesize that PWMI arises from maturation-dependent susceptibility of preOLs to death from hypoxia-ischemia. On-going studies with a neural stem cell-based culture system are defining the mechanisms of susceptibility of preOLs to oxidative stress in vitro. We have developed specialized expertise to study the responses of the OL lineage to oxidative stress under highly controlled conditions of low oxygen (hypoxia) or OGD.
Broughton, S.K., H Chen, R Mallinson, A. Riddle, S. Nagalla, C. T. Roberts, Jr, ,S.A. Back. Large-scale generation of highly enriched neural stem cell-derived oligodendroglial cultures: Maturation-dependent differences in insulin-like growth factor-mediated signal transduction, Journal of Neurochemistry, 100:628-638 (2007).
Back, S.A., X. Gan, Y. LI, P.A. Rosenberg, J.J. Volpe. Maturation-dependent vulnerability of oligodendroctyes to oxidative stress-induced death caused by glutathione depletion. Journal of Neuroscience 18(16):6241-6253 (1998).
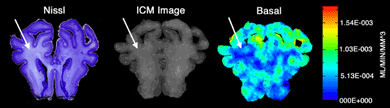
We have also developed in the 0.65 gestation fetal sheep a clinically relevant model of in utero global cerebral ischemia that allows integration of molecular, histological, physiological and neuroimaging approaches. We are generating three-dimensional reconstructions of fetal cerebral blood flow that are integrated with histopathology or neuroimaging data sets. Through the manipulation of these multi-modal 3-D data sets we expect to gain unprecedented access to questions central to the pathogenesis of brain injury during development.
Riddle, A., N.L. Luo, M. Manese, D.J. Beardsley, L. Green, D.A. Rorvik,, K.A. Kelly, C.H. Barlow, C.J. Kelly, A.R. Hohimer, S.A. Back. Spatial heterogeneity in oligodendrocyte maturation and not cerebral blood flow predicts fetal ovine periventricular white matter injury, Journal of Neuroscience, 26:3045-3055 (2006).
Back S.A., A. Riddle, A.R. Hohimer. Role of instrumented fetal sheep preparations in defining the pathogenesis of human periventricular white matter injury, Journal of Child Neurology, 21:582-589 (2006).
We are employing a perinatal rat model of hypoxia-ischemia to study how alterations in the extracellular matrix disrupt OL lineage progress and myelination. We have identified an astrocyte-derived form of hyaluonic acid (HA) that reversibly blocks the maturation of preOLs in vitro. In vivo, this form of HA causes arrested re-myelination after chemical injury to the myelin.
Back, S.A., Tuohy, T.M.F., H. Chen, N. Wallingford, A. Craig, J. Struve, N. Luo, F. Banine, Y. Liu, A. Chang, B.D. Trapp, B.F. Bebo, Jr., M.S. Rao, L.S. Sherman. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation, Nature Medicine 9:966-972, 2005.