Research
Overview: Delivering Next Generation Combination Immune Therapies for Cancer
Our research program employs proprietary nanoparticle platform to develop the next-generation combination immunotherapies for cancer. Our laboratory is a part of the Department of Biomedical Engineering, Oregon Health Science University (OHSU) School of Medicine. Enabled by our nano delivery platform, we bring complementary biological pathways together to achieve synergistic clinical benefit. Current focus is on developing IND-enabling stage therapeutic candidates that bridge the innate and adaptive immune systems by generating profound CD8+ T cell production
Cancer Immunotherapy
Yantasee Lab employs novel nanotechnology to develop next-generation combination immunotherapies for cancer. Learn more about Cancer Immunotherapy
Targeted Drug Delivery
We have developed nanoconstructs for targeted delivery of siRNA, drugs and imaging agents for the treatment and diagnosis of cancer. Learn more about Targeted Drug Delivery
Versatile Platform and Pipeline
Our proprietary Pdx-NP™ is innovative silica-based drug delivery technology that can load multiple cargo types at once Learn more about Versatile Platform and Pipeline.

Nanoparticle Engineering
Our proprietary Pdx-NP™ is innovative silica-based drug delivery technology that can load multiple cargo types at once (e.g., oligonucleotides, adjuvants, antibodies, chemotherapeutics, proteins).
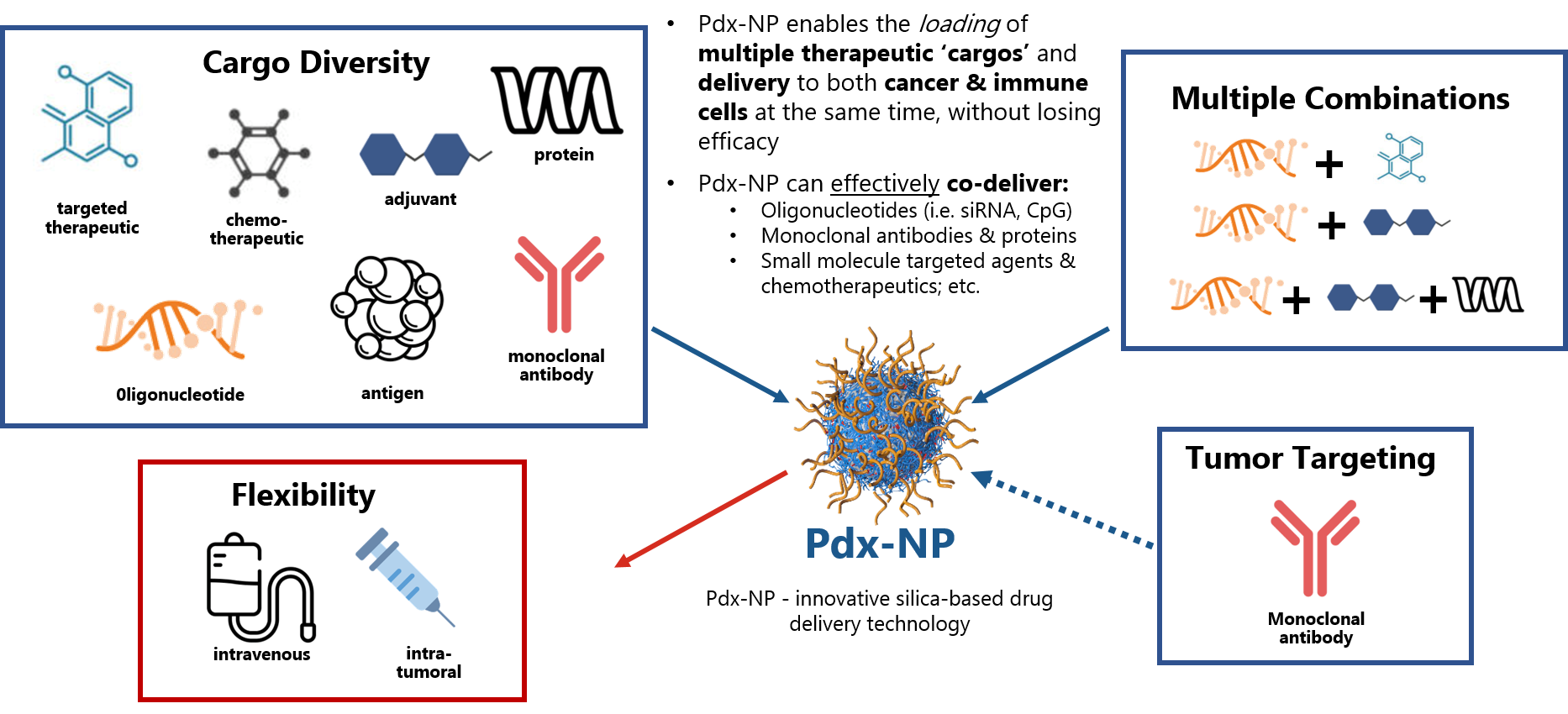
Pdx-NP: Achieves Superior Biological Outcomes
Pdx-NP (i.v.) delivers siRNA to breast tumor metastasis in lungs of mice
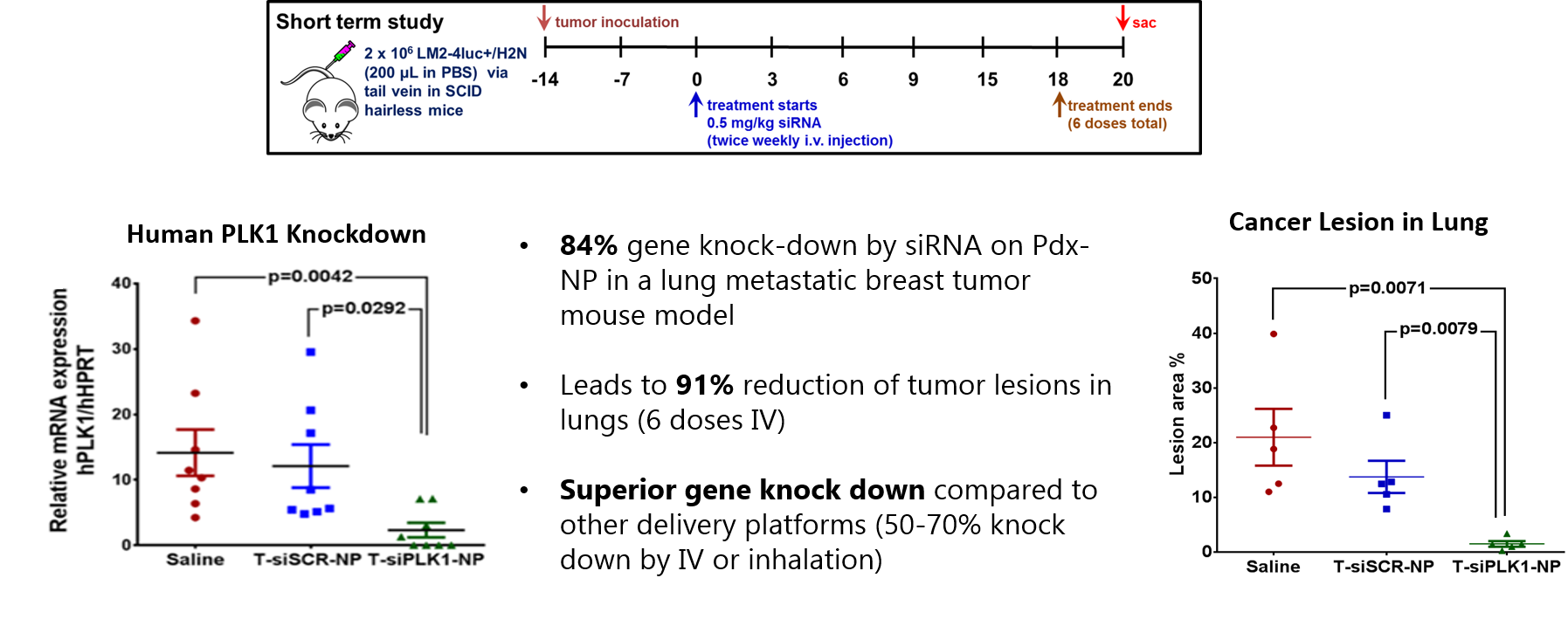
Pdx-NP retains intrinsic biological activity of each specific cargo (i.e., oligonucleotides, small molecules, etc.) ensuring optimal efficacy. Example with NP conjugated HER2 antibody as the targeting agent and delivering PLK1 siRNA.