Research
Overview
The AIMS lab is developing new technologies in the fields of artificial intelligence, computational diabetes, control theory, decision theory. Applications are in the areas of
- Closed loop artificial pancreas research and development
- Decision support
- Ubiquitous computing in the home
These projects also interrelate in various ways. A major goal of the lab is translation of our work for use to improve health.
Ongoing research projects
Development and evaluation of app-based certified diabetes education (AB-CDE) therapy for people living with T1D using MDI
Grant: Leona M. and Harry B. Helmsley Charitable Trust (AEDCN0362)
Co-Principal Investigator: Peter G. Jacobs and Leah M. Wilson
Project Start and End Dates: 11/1/2022 - 10/31/2025
Project Summary: Our goal is to integrate coaching and decision support technologies to improve glucose control in people with type 1 diabetes using multiple daily injection (MDI) therapy through a scalable, hierarchical therapy designed to deliver increasing levels of diabetes education and behavioral health interventions for those people who need it most.
Development of an open-source AI-based digital twin generator for replicating personalized glucose dynamics in people with type
Grant: JDRF Grant
Principal Investigator: Clara Mosquera-Lopez
Project Start and End Dates: 01/2023 – 12/2024
Project Summary: The purpose of this grant is to develop a new artificial intelligence platform for creating virtual twins. This will provide a tool for in silico testing and validation of new closed-loop systems, decision support algorithms, and therapeutic strategies.
Bias Equity and Inclusion in Artificial Intelligence for Medical Systems Internship (BE-In-AIMS)
Grant: OHSU DEI Funds
Principal Investigators: Peter Jacobs, Clara Mosquera-Lopez
Project Start and End Dates: 1/1/2022-12/31/2022
Project Summary: The goal of this grant is to create a new intern program called the Bias Equity and Inclusion in Artificial Intelligence for Medical Systems Internship (BE-In-AIMS) that will (1) help to recruit new underrepresented minorities to do AI research at OHSU and (2) encourage these researchers to explore how bias in health-related AI algorithms can contribute to negative health problems and (3) how to better train these algorithms to eliminate this bias.
Enabling fully automated closed loop control in type 1 diabetes through an artificial intelligence meal detection algorithm
Grant: R01DK129382
Program: NIH/NIDDK
Principal Investigators: Peter Jacobs and Leah Wilson
Project start and end dates: 8/20/2021 – 8/19/2025
Project summary: The objective of this project is to develop a fully automated artificial pancreas that automated delivery of both insulin and pramlintide hormones in combination with a forecasting algorithm that predicts when a person has consumed a meal.
Development and Evaluation of Machine Learning Models to Predict and Prevent Nocturnal Hypoglycemia in Type 1 Diabetes
Grant: R21DK128582
Program: NIH/NIDDK
Principal Investigator: Clara Mosquera-Lopez
Project start and end dates: 9/20/2021 – 9/19/2023
Project description: The goal for this project is to develop new glucose forecasting algorithms that can be used within a smart-phone app to help people with type 1 diabetes avoid nocturnal hypoglycemia by notifying them prior to their bedtime that they need to intervene.
Deciphering the Due Date - Using A.I. to Project and Improve Childbirth
Program: Oregon Health & Science University Foundation
PI: Erickson
Title: Deciphering the Due Date - Using A.I. to Project and Improve Childbirth
Dates of Project: 01/01/2022 – 12/31/2022
Project Summary: The goal of this project is to predict parturition using temperature data in humans.
Improving glycemic management in patients with type 1 diabetes using a context-aware automated insulin delivery system
Grant: 1 R01DK1225833-01
Program: NIH/NIDDK
Principal Investigator: Peter Jacobs and Jessica Castle
Project start and end dates: 7/15/2019-6/1/2023
Project summary: The objective of this project is to integrate contextual patterns of daily living as new inputs into an automated insulin delivery system to improve glycemic outcomes for people with type 1 diabetes.
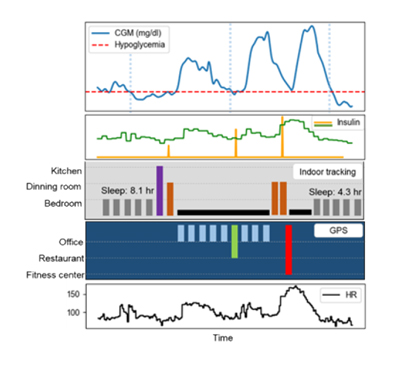
Improving glucose control with advanced technology designed for high risk patients with type 1 diabetes
Grant: NIH/NIDDK 1 R01DK120367-01
Program: NIH/NIDDK
Principal Investigator: Co-PIs Jacobs, Castle
Project Start and End Dates: 9/30/2018 - 6/30/2023
Project Summary: The objective of this project is to optimize the design and then evaluate a robust artificial pancreas system for use in patients with uncontrolled type 1 diabetes with HbA1C greater than 8% and compare HbA1C outcomes with these patients relative to a decision support system that utilizes continuous glucose monitoring (CGM) and multiple daily injection (MDI) therapy.
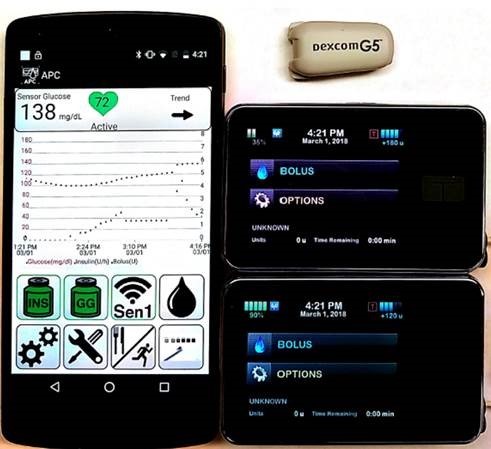
Exercise in diabetes initiative: The effect of exercise on glycemic control in type 1 diabetes (T1-DEXI)
Grant: Helmsley Charitable Trust
Program: HCT
Principal Investigator: Roy Beck (JAEB)
Co-investigator: Jacobs/Castle Sub-award co-PI
Project Start and End Dates: 11/30/2016 - present
Project Summary: A goal is to complete a protocol and a pilot study for a larger study that assesses how physical activity and nutrition impact glycemic control in people with type 1 diabetes. A second major goal is to complete the data acquisition hardware and software to support this study.
modular open source artificial pancreas platform called iPancreas
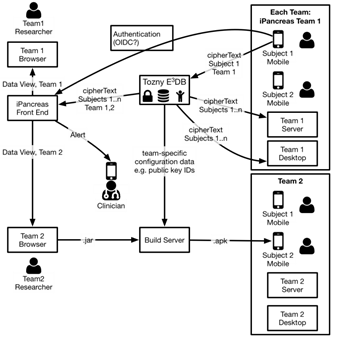
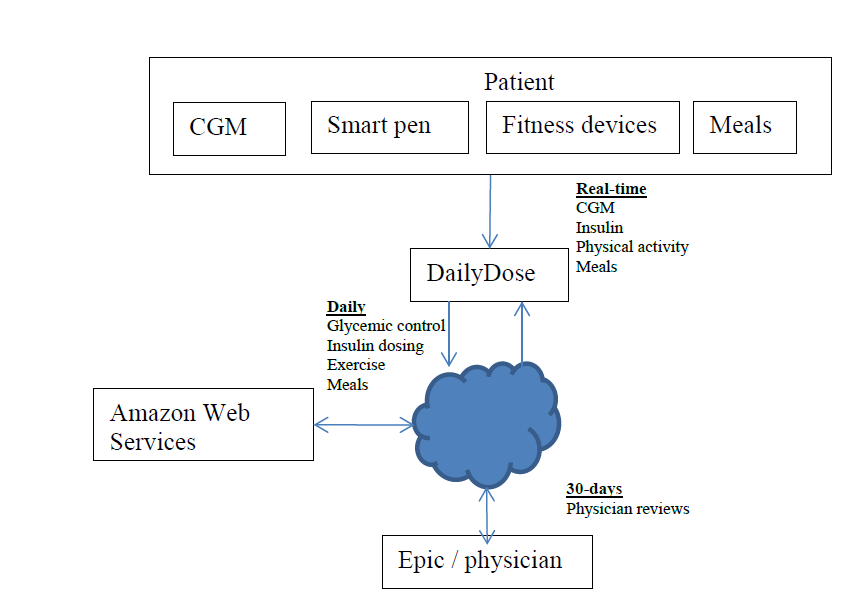
Design and evaluation of an MDI decision support system
Grant: 2018PG-T1D001
Program: Helmsley Charitable Trust
Principal Investigator: Jacobs, Castle
Project start and end dates: 5/1/2017 – 12/31/2022
Project summary: Funding for this project is to develop a decision support system called DailyDose that will enable improved glucose control for people with type 1 diabetes using multiple-daily injection (MDI) therapy with a smart pen technology.
Past projects
Grant: 2-SRA-2017-502-M-B
Program: Juvenile Diabetes Research Foundation
Principal Investigator: Jacobs, Castle
Project start and end dates: 8/1/2017 –7/31/2022
Project summary: In this project, we are accomplishing three major goals. The primary goal is to the research community a flexible, fully customizable open access artificial pancreas platform called iPancreas that is capable of integrating custom control algorithms as well as multiple input signals (glucose sensors, physical activity, skin impedance, and others) for automating delivery of insulin and glucagon to a person with type 1 diabetes. A second goal is to build a smart-phone AP system using iPancreas called AP-Active that integrates glucose sensor data as well as heart rate and accelerometer data to inform a control algorithm that will respond better to physical activity and help maintain euglycemia while preventing exercise-induced hypoglycemia. And third, we plan to evaluate AP-Active in 25 participants with type 1 diabetes in an 8-week randomized cross-over trial during which participants will use AP-Active for 4 weeks and will use a standard AP for 4 weeks.
![Flow diagram displaying Inputs [CGM, insulin, meals, exercise, time-based context]. Next stage of chart shows two flowchart paths: ConvLayers and LSTM. Last step of flow chart is glucose prediction.](/sites/default/files/2019-08/Jacobs%20Lab%20Project%20image%2011-%20lvgdataFlowChart.jpg)
Leveraging big data, virtual patient populations, and advanced machine learning to develop optimal personalized closed loop
Grant: 1-SRA-2019-820-S-B
Program: JDRF
Principal Investigator: Peter Jacobs
Project start and end dates: 7/1/2019-6/30/2020
Project summary: Funding for this project is to use the Tidepool big data set to design advanced machine learning and intelligent controls for personalized glucose control in type 1 diabetes.
Rare Diseases Clinical Research Consoria (RDCRC) for the Rare Diseases Clinical Research Network (RDCRN) – Pilot/Feasibility
Program: 30005550-0 NIH Children’s Research Institute (CRI)
PI: Gillingham
Dates of Project: 08/01/20 – 07/31/21
Project summary: We hypothesize the food photography estimate of energy intake will more closely match the total energy expenditure (TEE) than traditional 3-day diet records and be a more accurate method to measure dietary intake among subjects in the UCDC longitudinal study.
Novel Artificial Intelligence Algorithm to Automatically Detect, Diagnose, and Determine the Severity of Cardiac and Pulmonary D
Program: Oregon Health & Science University Foundation
PI: Schulman
Dates of Project: 06/01/20-09/30/21
Project summary: The goal of this project is to design new machine learning algorithms to predict heart murmurs from acoustic recordings of the heart.
Modeling of insulin and glucagon sensitivity during exercise in type 1 diabetes
Grant: NIH/NIDDK 1 R01 DK110175-01A1
Program: NIH/NIDDK
Principal Investigator: Jacobs, El Youssef
Project start and end dates: 4/1/2017 –3/31/22
Project summary: The purpose of this project is to investigate how the glucoregulatory system responds to different exercise paradigms (aerobic and anaerobic) in order to develop an artificial pancreas (AP) system for better control of insulin and glucagon delivery in type 1 diabetes (T1D).
Improving glycemic control through integration of ubiquitous wearable sensors within a
Comprehensive fall prevention and detection in MS
Grant: 1l01RX001831-01A1 (Cameron)
Program: VA RR&D VA Rehabilitation Research and Development Merit Award
Principal Investigator: Michelle Cameron
Project start and end dates: January 1 2016 – December 31 2020
Co-investigator: Peter Jacobs
Project Summary: The objective of this grant is to develop and evaluate a fall detection and localization system that my research team is currently developing. The evaluation will take place within a cohort of patients with MS who fall often.
Non-aqueous glucagon formulation to enable outpatient studies with a bi-hormonal pump
Grant: Juvenile Diabetes Research Foundation, Discovery & Development Partnership
Program: JDRF
Principal investigator: Castle, Jacobs, Petrelski
Project start and end dates: January 1st 2016 – December 31, 2017. Project summary: iPancreas: Internet based on-demand artificial pancreas app-generator to accelerate clinical trials research
Project summary:The goal of this project is to test a bi-hormonal closed-loop system utilizing two omnipods, one to deliver aspart insulin and one to deliver stabilized glucagon (formulated by Xeris) as compared to an insulin alone closed-loop system and as compared to standard of care (sensor-augmented pump) in the outpatient setting.
Mitigating risk in a closed loop system by exercise detection and miniaturization

Grant: 1DP3DK101044-01
Program: NIH National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
Principal Investigators: Peter Jacobs, Jessica R. Castle
Project Start and End Dates: September 30, 2013 to September 29, 2017
Project Summary: The objective of this project is to improve treatments for type 1 diabetes via optimization of a closed loop artificial pancreas system by 1) incorporating exercise detection to reduce the risk of hypoglycemia and 2) improving usability by reducing the number of system components.
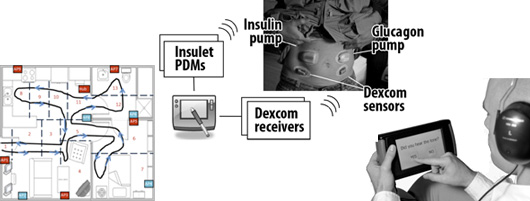
Components of the bi-hormonal artificial pancreas system: Two Tandem pumps, a Dexcom glucose sensor, and a Nexus smart phone running our control algorithm.
Protocols: A randomized four-way cross-over AP study
Unobtrusive measurement of sleep disordered breathing in the home

Grant: 2R01HL098621-04A1
Program: NIH National Heart, Blood and Lung Institute (NHLBI)
Principal Investigators: Chad Hagen, Peter Jacobs
Project Start and End Dates: January 1, 2014 to December 31, 2016
Project Summary: The purpose of this project is to use load cells to assess sleep apnea and other sleep disorders passively. Load cell developed to detect respiration and sleep apnea. This sensor gets placed on the rail of a bed frame. We use signal processing and machine learning to translate the sensed signal into an estimation of disordered breathing.
iPancreas: Internet based on-demand artificial pancreas app-generator to accelerate clinical trials research
Grant: 2017 Catalyst Award (Jacobs)
Program: OHSU OCTRI Pilot Award
Principal investigator:Peter G. Jacobs
Project start and end dates: July 1st 2016 – June 30th, 2017.
Project summary:The objective of this project is to translate the OHSU artificial pancreas (AP) technology for use by other research groups through the creation of iPancreas, an Internet-based on-demand AP app generator.
Position tracking and mobility assessment system for indoor monitoring of elders
Grant: 2R42AG035400-02A1
Program: NIH Small Business Technology Transfer (STTR)
Principal Investigator: Eric A. Wan
Co-investigator: Peter Jacobs
Project Start and End Dates: Sept 1 2010 – May 31, 2016
Project Summary: This phase-2 STTR will commercialize a Position Tracking and Mobility Assessment System targeted specifically for elderly patients within their home environment.

Comprehensive ototoxicity monitoring program for VA: A randomized trial

Grant: RX000239
Program: VA Rehabilitation Research and Development Merit Award
Principal Investigators: Marilyn Dille, Dawn Konrad-Martin
Project Start and End Dates: April 1, 2014 to March 31, 2018
Co-Investigator: Peter Jacobs
Project Summary: The proposed study is designed to establish that ototoxicity monitoring when introduced as a comprehensive program of evidence-based protocols will improve Veteran hearing outcomes and quality of life by influencing therapeutic planning and improving access to timely audiological rehabilitation services.
A passive tag-free approach to localization and activity monitoring
Grant: ETAC-12-239042
Program: Alzheimer's Association 2012 Everyday Technologies for Alzheimer's Care
Principal Investigators: Eric Wan, Peter Jacobs
Project Start and End Dates: November 1, 2012 to October 1, 2014
Grant: 1R43AG049573 –01A1 (Jacobs, Wan)
Program: NIH/NIA National Institute for aging
Principal investigator: Peter Jacobs
Project start and End Dates: Sept 1 2010 – May 31, 2016
Co-investigator: Michelle Cameron
Project summary: In-home monitoring system for assessing gait using wall-mounted Radio Frequency transceivers. The objective of this project is to develop new ways of assessing gait using Radio Frequency fingerprinting and RSSI signals.
Project Summary: The objective of this grant is to research and develop a Position Tracking and Mobility Assessment System targeted specifically for monitoring patients with mild cognitive impairment / Alzheimer’s Disease within their home environment.
Mobile monitoring of chemotherapy-induced peripheral neuropathy

Program: OHSU Pilot Project
Principal Investigator: Kerri M. Winters-Stone
Co-investigator: Peter Jacobs
Project Start and End Dates: Sept 1, 2014-Aug 30 2016
Project Summary: We are developing a mobile phone-based system that could accurately quantify chemotherapy-induced peripheral neuropathy symptoms and functional changes using a smartphone application interface that is easy to administer by a clinician or a non-expert such as by a patient herself and which could be broadly implemented in multiple settings and populations. For the proposed study we want to determine the feasibility and acceptability of the mobile phone system by clinicians, nurses, physical therapists and patients. The feasibility and acceptability data will be used to refine the user interface, data collection protocols, signal processing during a neuropathy test, balance and sway test, and walking test and other preferences and features of the system to maximize utilization potential in the clinic and home settings. We expect that following this feasibility study the system will be ready for near-term, widespread implementation.